Traumatic brain injury-induced inflammatory changes in the olfactory bulb disrupt neuronal networks leading to olfactory dysfunction
- PMID: 37557959
- PMCID: PMC10910858
- DOI: 10.1016/j.bbi.2023.08.004
Traumatic brain injury-induced inflammatory changes in the olfactory bulb disrupt neuronal networks leading to olfactory dysfunction
Abstract
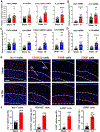
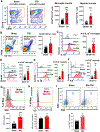
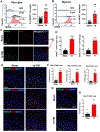
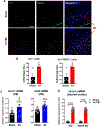
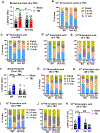
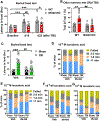
Approximately 20-68% of traumatic brain injury (TBI) patients exhibit trauma-associated olfactory deficits (OD) which can compromise not only the quality of life but also cognitive and neuropsychiatric functions. However, few studies to date have examined the impact of experimental TBI on OD. The present study examined inflammation and neuronal dysfunction in the olfactory bulb (OB) and the underlying mechanisms associated with OD in male mice using a controlled cortical impact (CCI) model. TBI caused a rapid inflammatory response in the OB as early as 24 h post-injury, including elevated mRNA levels of proinflammatory cytokines, increased numbers of microglia and infiltrating myeloid cells, and increased IL1β and IL6 production in these cells. These changes were sustained for up to 90 days after TBI. Moreover, we observed significant upregulation of the voltage-gated proton channel Hv1 and NOX2 expression levels, which were predominantly localized in microglia/macrophages and accompanied by increased reactive oxygen species production. In vivo OB neuronal firing activities showed early neuronal hyperexcitation and later hypo-neuronal activity in both glomerular layer and mitral cell layer after TBI, which were improved in the absence of Hv1. In a battery of olfactory behavioral tests, WT/TBI mice displayed significant OD. In contrast, neither Hv1 KO/TBI nor NOX2 KO/TBI mice showed robust OD. Finally, seven days of intranasal delivery of a NOX2 inhibitor (NOX2ds-tat) ameliorated post-traumatic OD. Collectively, these findings highlight the importance of OB neuronal networks and its role in TBI-mediated OD. Thus, targeting Hv1/NOX2 may be a potential intervention for improving post-traumatic anosmia.
Keywords: Hv1; NOX2; Neuroinflammation; Olfactory dysfunction; Traumatic brain injury.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

