Single-cell analysis of dorsal root ganglia reveals metalloproteinase signaling in satellite glial cells and pain
- PMID: 37557960
- PMCID: PMC10530626
- DOI: 10.1016/j.bbi.2023.08.005
Single-cell analysis of dorsal root ganglia reveals metalloproteinase signaling in satellite glial cells and pain
Abstract
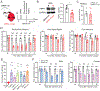
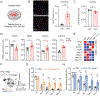
Satellite glial cells (SGCs) are among the most abundant non-neuronal cells in dorsal root ganglia (DRGs) and closely envelop sensory neurons that detect painful stimuli. However, little is still known about their homeostatic activities and their contribution to pain. Using single-cell RNA sequencing (scRNA-seq), we were able to obtain a unique transcriptional profile for SGCs. We found enriched expression of the tissue inhibitor metalloproteinase 3 (TIMP3) and other metalloproteinases in SGCs. Small interfering RNA and neutralizing antibody experiments revealed that TIMP3 modulates somatosensory stimuli. TIMP3 expression decreased after paclitaxel treatment, and its rescue by delivery of a recombinant TIMP3 protein reversed and prevented paclitaxel-induced pain. We also established that paclitaxel directly impacts metalloproteinase signaling in cultured SGCs, which may be used to identify potential new treatments for pain. Therefore, our results reveal a metalloproteinase signaling pathway in SGCs for proper processing of somatosensory stimuli and potential discovery of novel pain treatments.
Keywords: Metalloproteinases; Neuropathic pain; Satellite glial cells; Single-cell RNA sequencing.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

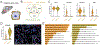


References
-
- Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW, 2018. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

