Interpreting the Results of Trials of BCG Vaccination for Protection Against COVID-19
- PMID: 37558650
- PMCID: PMC10640778
- DOI: 10.1093/infdis/jiad316
Interpreting the Results of Trials of BCG Vaccination for Protection Against COVID-19
Abstract
BCG vaccination has beneficial off-target ("nonspecific") effects on nonmycobacterial infections. On this premise, trials set out to investigate whether BCG provides off-target protection against coronavirus disease 2019 (COVID-19). A literature search identified 11 randomized "BCG COVID-19" trials, with conflicting results. These trials and the differences in their study design are discussed using the PICOT (participants, intervention, control, outcome, and timing) framework to highlight the factors that likely explain their inconsistent findings. These include participant age, sex and comorbid conditions, BCG vaccination strain and dose, outcome measure and duration of follow-up. Understanding how to control these factors to best exploit BCG's off-target effects will be important in designing future trials and intervention strategies.
Keywords: BCG vaccination; COVID-19; SARS-CoV-2; off-target effects.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. N. L. M., L. F. P., and N. C. are investigators on the BRACE trial. C. C. A. N. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


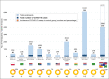

References
-
- World Health Organization . BCG vaccines: WHO position paper—February 2018. Wkly Epidemiol Rec 2018; 93:73–96.
-
- Pollard AJ, Finn A, Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child 2017; 102:1077–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

