Intrinsic dopamine and acetylcholine dynamics in the striatum of mice
- PMID: 37558873
- PMCID: PMC11577287
- DOI: 10.1038/s41586-023-05995-9
Intrinsic dopamine and acetylcholine dynamics in the striatum of mice
Abstract
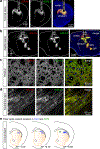
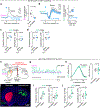
External rewards such as food and money are potent modifiers of behaviour1,2. Pioneering studies established that these salient sensory stimuli briefly interrupt the tonic discharge of neurons that produce the neuromodulators dopamine (DA) and acetylcholine (ACh): midbrain DA neurons (DANs) fire a burst of action potentials that broadly elevates DA in the striatum3,4 at the same time that striatal cholinergic interneurons (CINs) produce a characteristic pause in firing5,6. These phasic responses are thought to create unique, temporally limited conditions that motivate action and promote learning7-11. However, the dynamics of DA and ACh outside explicitly rewarded situations remain poorly understood. Here we show that extracellular DA and ACh levels fluctuate spontaneously and periodically at a frequency of approximately 2 Hz in the dorsal striatum of mice and maintain the same temporal relationship relative to one another as that evoked by reward. We show that this neuromodulatory coordination does not arise from direct interactions between DA and ACh within the striatum. Instead, we provide evidence that periodic fluctuations in striatal DA are inherited from midbrain DANs, while striatal ACh transients are driven by glutamatergic inputs, which act to locally synchronize the spiking of CINs. Together, our findings show that striatal neuromodulatory dynamics are autonomously organized by distributed extra-striatal afferents. The dominance of intrinsic rhythms in DA and ACh offers new insights for explaining how reward-associated neural dynamics emerge and how the brain motivates action and promotes learning from within.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures
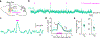
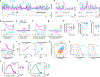









References
-
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57, 87–115 (2006). - PubMed
-
- Mirenowicz J. & Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379, 449–451 (1996). - PubMed
-
- Morris G, Arkadir D, Nevet A, Vaadia E. & Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43, 133–143 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

