Mitochondrial integrated stress response controls lung epithelial cell fate
- PMID: 37558881
- PMCID: PMC10447247
- DOI: 10.1038/s41586-023-06423-8
Mitochondrial integrated stress response controls lung epithelial cell fate
Abstract
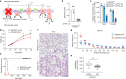
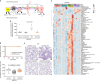
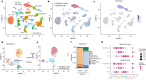
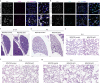
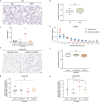
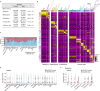
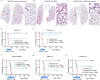
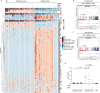
Alveolar epithelial type 1 (AT1) cells are necessary to transfer oxygen and carbon dioxide between the blood and air. Alveolar epithelial type 2 (AT2) cells serve as a partially committed stem cell population, producing AT1 cells during postnatal alveolar development and repair after influenza A and SARS-CoV-2 pneumonia1-6. Little is known about the metabolic regulation of the fate of lung epithelial cells. Here we report that deleting the mitochondrial electron transport chain complex I subunit Ndufs2 in lung epithelial cells during mouse gestation led to death during postnatal alveolar development. Affected mice displayed hypertrophic cells with AT2 and AT1 cell features, known as transitional cells. Mammalian mitochondrial complex I, comprising 45 subunits, regenerates NAD+ and pumps protons. Conditional expression of yeast NADH dehydrogenase (NDI1) protein that regenerates NAD+ without proton pumping7,8 was sufficient to correct abnormal alveolar development and avert lethality. Single-cell RNA sequencing revealed enrichment of integrated stress response (ISR) genes in transitional cells. Administering an ISR inhibitor9,10 or NAD+ precursor reduced ISR gene signatures in epithelial cells and partially rescued lethality in the absence of mitochondrial complex I function. Notably, lung epithelial-specific loss of mitochondrial electron transport chain complex II subunit Sdhd, which maintains NAD+ regeneration, did not trigger high ISR activation or lethality. These findings highlight an unanticipated requirement for mitochondrial complex I-dependent NAD+ regeneration in directing cell fate during postnatal alveolar development by preventing pathological ISR induction.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

