Sex differences in metabolic phenotype and hypothalamic inflammation in the 3xTg-AD mouse model of Alzheimer's disease
- PMID: 37559092
- PMCID: PMC10410820
- DOI: 10.1186/s13293-023-00536-5
Sex differences in metabolic phenotype and hypothalamic inflammation in the 3xTg-AD mouse model of Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is notably associated with cognitive decline resulting from impaired function of hippocampal and cortical areas; however, several other domains and corresponding brain regions are affected. One such brain region is the hypothalamus, shown to atrophy and develop amyloid and tau pathology in AD patients. The hypothalamus controls several functions necessary for survival, including energy and glucose homeostasis. Changes in appetite and body weight are common in AD, often seen several years prior to the onset of cognitive symptoms. Therefore, altered metabolic processes may serve as a biomarker for AD, as well as a target for treatment, considering they are likely both a result of pathological changes and contributor to disease progression. Previously, we reported sexually dimorphic metabolic disturbances in ~ 7-month-old 3xTg-AD mice, accompanied by differences in systemic and hypothalamic inflammation.
Methods: In the current study, we investigated metabolic outcomes and hypothalamic inflammation in 3xTg-AD males and females at 3, 6, 9, and 12 months of age to determine when these sex differences emerge.
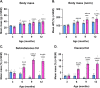
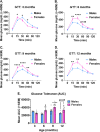
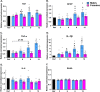
Results: In agreement with our previous study, AD males displayed less weight gain and adiposity, as well as reduced blood glucose levels following a glucose challenge, compared to females. These trends were apparent by 6-9 months of age, coinciding with increased expression of inflammatory markers (Iba1, GFAP, TNF-α, and IL-1β) in the hypothalamus of AD males.
Conclusions: These findings provide additional evidence for sex-dependent effects of AD pathology on energy and glucose homeostasis, which may be linked to hypothalamic inflammation.
Keywords: Alzheimer’s disease; Gender; Hypothalamus; Inflammation; Metabolic; Sex.
Plain language summary
Alzheimer’s disease (AD), often associated with memory loss, can also affect other parts of the brain and body, resulting in several other symptoms. Changes in appetite and body weight are commonly seen in people with AD, often before they start showing signs of memory loss. These metabolism-related changes are likely due in part to AD affecting a part of the brain called the hypothalamus, which controls important functions like energy balance (calories in vs. calories out) and blood sugar levels. This study aimed to examine whether changes in metabolism and the hypothalamus could serve as early signs of AD, and even help in treating the disease. We also wanted to see if these changes were influenced by biological sex, as two-thirds of AD patients are women, and our previous studies showed many differences between males and females. In this study, we observed male and female mice at different ages to see when these changes began to appear. We found that male AD mice gained less weight, had less body fat, and had better blood sugar control, compared to female AD mice. These differences became noticeable at the same age that we noticed signs of increased inflammation in the hypothalamus of male mice. These findings suggest that AD affects males and females differently, particularly in terms of energy balance and blood sugar control, and this might be related to inflammation in the hypothalamus. This research could provide valuable insights into understanding, diagnosing, and treating Alzheimer’s disease.
© 2023. Society for Women's Health Research and BioMed Central Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. Global status report on the public health response to dementia. 2021.
-
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dementia. 2013;9(1):63–75.e2. - PubMed
-
- Ishii M, Wang G, Racchumi G, Dyke JP, Iadecola C. Transgenic mice overexpressing amyloid precursor protein exhibit early metabolic deficits and a pathologically low leptin state associated with hypothalamic dysfunction in arcuate neuropeptide Y neurons. J Neurosci. 2014;34(27):9096–9106. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

