Higher levels of minimal residual disease in peripheral blood than bone marrow before 1st and 2nd relapse/regrowth in a patient with high‑risk neuroblastoma: A case report
- PMID: 37559575
- PMCID: PMC10407720
- DOI: 10.3892/ol.2023.13955
Higher levels of minimal residual disease in peripheral blood than bone marrow before 1st and 2nd relapse/regrowth in a patient with high‑risk neuroblastoma: A case report
Abstract
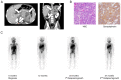
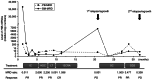
More than half of patients with high-risk neuroblastoma (HR-NB) experience relapse/regrowth due to the activation of chemoresistant minimal residual disease (MRD). MRD in patients with HR-NB can be evaluated by quantitating neuroblastoma-associated mRNAs (NB-mRNAs) in bone marrow (BM) and peripheral blood (PB) samples. Although several sets of NB-mRNAs have been shown to possess a prognostic value for MRD in BM samples (BM-MRD), MRD in PB samples (PB-MRD) is considered to be low and difficult to evaluate. The present report describes an HR-NB case presenting higher PB-MRD than BM-MRD before 1st and 2nd relapse/regrowth. A 3-year-old female presented with an abdominal mass, was diagnosed with HR-NB, and treated according to the nationwide standard protocol for HR-NB. Following systemic induction and consolidation therapy with local therapy, the patient achieved complete remission but experienced a 1st relapse/regrowth 6 months after maintenance therapy. The patient partially responded to salvage chemotherapy and anti-GD2 immunotherapy but had a 2nd relapse/regrowth 14 months after the 1st relapse/regrowth. Consecutive PB-MRD and BM-MRD monitoring revealed that PB-MRD was lower than BM-MRD at diagnosis (100 times) and 1st and 2nd relapse/regrowth (1,000 and 3 times) but became higher than BM-MRD before 1st and 2nd relapse/regrowth. The present case highlights that PB-MRD can become higher than BM-MRD before relapse/regrowth of patients with HR-NB.
Keywords: bone marrow; minimal residual disease; neuroblastoma; neuroblastoma-associated mRNAs; peripheral blood; relapse/regrowth.
Copyright © 2023, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
