Divergent adaptive immune responses define two types of long COVID
- PMID: 37559726
- PMCID: PMC10408302
- DOI: 10.3389/fimmu.2023.1221961
Divergent adaptive immune responses define two types of long COVID
Abstract
Background: The role of adaptive immune responses in long COVID remains poorly understood, with contrasting hypotheses suggesting either an insufficient antiviral response or an excessive immune response associated with inflammatory damage. To address this issue, we set to characterize humoral and CD4+ T cell responses in long COVID patients prior to SARS-CoV-2 vaccination.
Methods: Long COVID patients who were seropositive (LC+, n=28) or seronegative (LC-, n=23) by spike ELISA assay were recruited based on (i) an initial SARS-CoV-2 infection documented by PCR or the conjunction of three major signs of COVID-19 and (ii) the persistence or resurgence of at least 3 symptoms for over 3 months. They were compared to COVID patients with resolved symptoms (RE, n=29) and uninfected control individuals (HD, n=29).
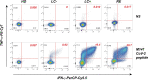
Results: The spectrum of persistent symptoms proved similar in both long COVID groups, with a trend for a higher number of symptoms in the seronegative group (median=6 vs 4.5; P=0.01). The use a highly sensitive S-flow assay enabled the detection of low levels of SARS-CoV-2 spike-specific IgG in 22.7% of ELISA-seronegative long COVID (LC-) patients. In contrast, spike-specific IgG levels were uniformly high in the LC+ and RE groups. Multiplexed antibody analyses to 30 different viral antigens showed that LC- patients had defective antibody responses to all SARS-CoV-2 proteins tested but had in most cases preserved responses to other viruses. A sensitive primary T cell line assay revealed low but detectable SARS-CoV-2-specific CD4 responses in 39.1% of LC- patients, while response frequencies were high in the LC+ and RE groups. Correlation analyses showed overall strong associations between humoral and cellular responses, with exceptions in the LC- group.
Conclusions: These findings provide evidence for two major types of antiviral immune responses in long COVID. Seropositive patients showed coordinated cellular and humoral responses at least as high as those of recovered patients. In contrast, ELISA-seronegative long COVID patients showed overall low antiviral responses, with detectable specific CD4+ T cells and/or antibodies in close to half of patients (52.2%). These divergent findings in patients sharing a comparable spectrum of persistent symptoms raise the possibility of multiple etiologies in long COVID.
Keywords: SARS-CoV-2; T cell; common cold coronavirus; humoral immune response; long COVID; seronegative and seropositive.
Copyright © 2023 Kervevan, Staropoli, Slama, Jeger-Madiot, Donnadieu, Planas, Pietri, Loghmari-Bouchneb, Alaba Tanah, Robinot, Boufassa, White, Salmon-Ceron and Chakrabarti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Gottlieb M, Wang R, Yu H, Spatz ES, Montoy JC, Rodriguez R, et al. . Severe fatigue and persistent symptoms at three months following SARS-CoV-2 infections during the pre-delta, delta, and omicron time periods: a multicenter prospective cohort study. Clin Infect Dis (2023) 76:1930–1941. doi: 10.1093/cid/ciad045 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

