Hepatitis C accelerates extrahepatic cholangiocarcinoma risk: a joint study of hospital-based cases and nationwide population-based cohorts in a viral hepatitis-endemic area
- PMID: 37559993
- PMCID: PMC10408483
Hepatitis C accelerates extrahepatic cholangiocarcinoma risk: a joint study of hospital-based cases and nationwide population-based cohorts in a viral hepatitis-endemic area
Abstract
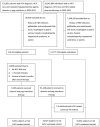
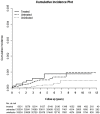
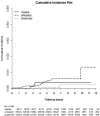
Hepatitis C virus (HCV) infection causes many cancers, including intrahepatic cholangiocarcinoma. Whether it increases the risk of extrahepatic cholangiocarcinoma (ECC) is unknown. A 10-year nationwide population-based cohort study of the Taiwan National Health Insurance Research Database (TNHIRD) was conducted. ECC was defined by ICD-9-CM code 156 or ICD-O-3 code C23-24. Risk factors and HCV core protein expression were surveyed in patients with ECC from a tertiary-care center. Out of 11,892,067 patients, three propensity score-matched TNHIRD cohorts were matched at a 1:4:4 ratio: HCV-treated (8,331 patients with interferon-based therapy >6 months), HCV-untreated (n=33,324), and HCV-uninfected cohorts (n=33,324). The cumulative incidence of ECC [HCV-treated: 0.088%, 95% confidence interval (CI): 0.035-0.198%; HCV-untreated: 0.095%, 0.047-0.179%; HCV-uninfected: 0.048%, 0.017-0.119%] was lowest in the HCV-uninfected cohort (P=0.0285) but was not different between the treated and untreated cohorts (P=0.5436). HCV infection [HCV-treated cohort: hazard ratio (HR): 3.618, 95% CI HR: 1.253-10.451; HCV-untreated cohort: 2.593, 95% CI HR: 1.077-6.241; reference: HCV-uninfected cohort] and age ≥49 years (HR: 5.139, 95% CI HR: 1.613-16.369) were associated with ECC development. Among the 855 hospitalized ECC patients (males: 57%; baseline age: 63.09±11.75 years, 2008-2018), the HCV Ab-positive rate was 8.4%. The HCV Ab-positive patients were more frequently female than their counterparts (66.7% vs. 40.8%, P=0.009). No HCV core-positive cells were found in the ECC tissues. In conclusion, HCV infection and age ≥49 years are potential risk factors for ECC. The HCV-associated ECC risk might not be reversed by interferon-based anti-HCV therapy nor associated with in situ HCV core-related carcinogenesis.
Keywords: GB; HCV; age; extrahepatic cholangiocarcinoma.
AJCR Copyright © 2023.
Conflict of interest statement
None.
Figures

References
-
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. - PubMed
LinkOut - more resources
Full Text Sources
