Challenges and prospects of CSF1R targeting for advanced malignancies
- PMID: 37560003
- PMCID: PMC10408490
Challenges and prospects of CSF1R targeting for advanced malignancies
Abstract
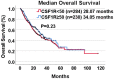
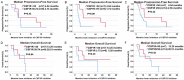
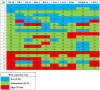
CSF1R expression modulates tumor-associated macrophages, making CSF1R blockade an appealing immune-modulating therapeutic target. We evaluated the correlation between CSF1R tumor RNA expression and outcome (pan-cancer setting). RNA expression was ranked as a percentile (0-100) using a standardized internal reference population (735 tumors; 35 histologies). Among 514 patients, there was no difference in survival from biopsy between high and low CSF1R expressors (< 50 percentile versus ≥ 50 percentile rank). There was also no significant difference in median progression-free or overall survival (from treatment) based on CSF1R expression in 21 patients who received CSF1R inhibitors (all p values ≥ 0.08). Concurrent upregulation of ≥ 2 additional immune checkpoint markers (e.g. PD-L1, BTLA, CTLA4, LAG3, TIM3) was observed in all tumor samples with CSF1R expression ≥ 50th percentile. Pending further large prospective studies, patients with high tumor CSF1R expression may need treatment that co-targets the specific immune checkpoint pathways activated in order to impact outcome.
Keywords: CSF1R; biomarker; cancer; checkpoints; targeted therapy.
AJCR Copyright © 2023.
Conflict of interest statement
Ann Moeller, Suzanna Lee, and Hitendra Patel declare that they have no competing interests. Sarabjot Pabla, Mary K. Nesline, Jeffrey Conroy are employees of Omniseq, Inc. Jason K. Sicklick receives research funding from Amgen, Foundation Medicine is a consultant for Deciphera, Ethicon and speaker for Bayer, Deciphera, Foundation Medicine, La Hoffman-Roche, Merck, QED. Gregory P. Botta serves on the Advisory Board of Natera and as a consultant to TumorGen Inc. and CEND Therapeutics.Shumei Kato serves as a consultant for Foundation Medicine, NeoGenomics and CureMatch. He receives speaker’s fee from Roche and advisory board for Pfizer. He has research funding from ACT Genomics, Sysmex, Konica Minolta and OmniSeq.Jacob J. Adashek serves on the advisory board of CureMatch, Inc. Razelle Kurzrock has received research funding from Biological Dynamics, Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, Daiichi Sankyo, Inc., EISAI, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc., CureMetrix, and IDbyDNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch.
Figures
Similar articles
-
Expression of TIM3/VISTA checkpoints and the CD68 macrophage-associated marker correlates with anti-PD1/PDL1 resistance: implications of immunogram heterogeneity.Oncoimmunology. 2020 Feb 17;9(1):1708065. doi: 10.1080/2162402X.2019.1708065. eCollection 2020. Oncoimmunology. 2020. PMID: 32117584 Free PMC article.
-
Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas.Gastroenterology. 2017 Oct;153(4):1107-1119.e10. doi: 10.1053/j.gastro.2017.06.017. Epub 2017 Jun 23. Gastroenterology. 2017. PMID: 28648905
-
Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade.Gut. 2019 Sep;68(9):1653-1666. doi: 10.1136/gutjnl-2019-318419. Epub 2019 Mar 22. Gut. 2019. PMID: 30902885
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
-
Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC).J Immunother Cancer. 2018 May 16;6(1):39. doi: 10.1186/s40425-018-0349-3. J Immunother Cancer. 2018. PMID: 29769148 Free PMC article. Review.
Cited by
-
The crosstalk between lung cancer and the bone marrow niche fuels emergency myelopoiesis.Front Immunol. 2024 Aug 1;15:1397469. doi: 10.3389/fimmu.2024.1397469. eCollection 2024. Front Immunol. 2024. PMID: 39148724 Free PMC article. Review.
-
Myeloid-derived suppressor cells attenuate the antitumor efficacy of radiopharmaceutical therapy using 90Y-NM600 in combination with androgen deprivation therapy in murine prostate tumors.J Immunother Cancer. 2024 Apr 24;12(4):e008760. doi: 10.1136/jitc-2023-008760. J Immunother Cancer. 2024. PMID: 38663936 Free PMC article.
-
Identification of Selective Imidazopyridine CSF1R Inhibitors.ACS Med Chem Lett. 2024 Apr 30;15(5):722-730. doi: 10.1021/acsmedchemlett.4c00110. eCollection 2024 May 9. ACS Med Chem Lett. 2024. PMID: 38746878 Free PMC article.
-
Cholesterol efflux protein, ABCA1, supports anti-cancer functions of myeloid immune cells.bioRxiv [Preprint]. 2025 Feb 19:2025.02.19.638515. doi: 10.1101/2025.02.19.638515. bioRxiv. 2025. PMID: 40027727 Free PMC article. Preprint.
-
Sequence of androgen receptor-targeted vaccination with androgen deprivation therapy affects anti-prostate tumor efficacy.J Immunother Cancer. 2024 May 20;12(5):e008848. doi: 10.1136/jitc-2024-008848. J Immunother Cancer. 2024. PMID: 38772685 Free PMC article.
References
-
- Kumari A, Silakari O, Singh RK. Recent advances in colony stimulating factor-1 receptor/c-FMS as an emerging target for various therapeutic implications. Biomed Pharmacother. 2018;103:662–679. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
