Analysis of Patients' Characteristics and Treatment Profile of People Who Use Drugs (PWUDs) with and without a Co-Diagnosis of Viral Hepatitis C: A Real-World Retrospective Italian Analysis
- PMID: 37560130
- PMCID: PMC10408688
- DOI: 10.2147/TCRM.S409134
Analysis of Patients' Characteristics and Treatment Profile of People Who Use Drugs (PWUDs) with and without a Co-Diagnosis of Viral Hepatitis C: A Real-World Retrospective Italian Analysis
Abstract
Purpose: Hepatitis C virus (HCV) spreads from contact with blood of an infected person. HCV infections are common among people who use drugs (PWUDs), when sharing needles, syringes, or other equipment for injected drugs. The advent of pangenotypic direct-antiviral agents (DAA) in 2017 transformed the treatment landscape for HCV, but PWUDs remain a complex and hard-to-treat population with high risk of HCV reinfection. The aim of this real-world analysis was to characterize the demographic and clinical features of PWUDs in Italy, also focusing on comorbidity profile, treatment with DAAs, resource consumptions for the National Health System (NHS).
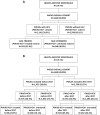
Patients and methods: During 01/2011-06/2020, administrative databases of Italian healthcare entities, covering 3,900,000 individuals, were browsed to identify PWUDs with or without HCV infection. Among HCV+ patients, a further stratification was made into treated and untreated with DAAs. The date of PWUD or HCV first diagnosis or DAA first prescription was considered as index-date. Patients were then followed-up for one year. Alcohol-dependency was also investigated.
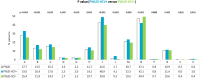
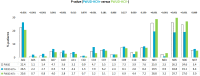
Results: Total 3690 PWUDs were included, of whom 1141 (30.9%) PWUD-HCV+ and 2549 (69.1%) PWUD-HCV-. HCV-positive were significantly older (43.6 vs 38.5 years, p < 0.001), had a worse comorbidity profile (Charlson-index: 0.8 vs 0.4, p < 0.001), and high rates of psychiatric, respiratory, dermatological, musculoskeletal diseases and genitourinary (sexually transmitted) infections. Moreover, they received more drug prescriptions (other than DAAs, like anti-acids, antiepileptics, psycholeptics) and had undergone more frequent hospitalization, predominantly for hepatobiliary, respiratory system and mental disorders. DDA-untreated had significantly higher Charlson-index than DAA-treated (0.9 vs 0.6, p = 0.003). Alcoholism was found in 436 (11.8%) cases.
Conclusion: This Italian real-world analysis suggests that PWUDs with HCV infection, especially those untreated with DAAs, show an elevated drug consumption due to their complex clinical profile. These findings could help to ameliorate the healthcare interventions on PWUDs with HCV infection.
Keywords: alcohol dependency; drug abuse; hepatitis C virus; real-world evidence.
© 2023 Nava et al.
Conflict of interest statement
A.M. declares teaching and speaking and research support: Angelini, Gilead Sciences, Polifarma. F.F and C.H are employees of Gilead Sciences. M.P. reports grants, personal fees, non-financial support from Gilead, AbbVie; personal fees from Merck, during the conduct of the study; personal fees from Angelini, Astra Zeneca, GSK, Menarini, Janssen, Roche, and Novartis, outside the submitted work. All other authors report no conflicts of interest in this work.
Figures
Similar articles
-
A Real-World Analysis of the Population with Hepatitis C Virus Infection Affected by Type 2 Diabetes in Italy: Patients' Characteristics, Comorbidity Profiles and Treatment Patterns.Medicina (Kaunas). 2025 Mar 28;61(4):614. doi: 10.3390/medicina61040614. Medicina (Kaunas). 2025. PMID: 40282905 Free PMC article.
-
Factors Enhancing Treatment of Hepatitis C Virus-Infected Italian People Who Use Drugs: The CLEO-GRECAS Experience.Am J Gastroenterol. 2021 Jun 1;116(6):1248-1255. doi: 10.14309/ajg.0000000000001147. Am J Gastroenterol. 2021. PMID: 34074828
-
Changes in the profile and therapeutic care of people who use drugs with HCV mono-infection: a retrospective study between 2015 and 2019 from a monocentric tertiary referent center in France.Eur J Gastroenterol Hepatol. 2022 May 1;34(5):560-566. doi: 10.1097/MEG.0000000000002307. Eur J Gastroenterol Hepatol. 2022. PMID: 35421021
-
Real-World Efficacy and Safety of Pangenotypic Direct-Acting Antivirals Against Hepatitis C Virus Infection.Rev Recent Clin Trials. 2019;14(3):173-182. doi: 10.2174/1574887114666190306154650. Rev Recent Clin Trials. 2019. PMID: 30848211 Review.
-
Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System.Ann Intern Med. 2017 Jun 6;166(11):792-798. doi: 10.7326/M17-0377. Epub 2017 Apr 25. Ann Intern Med. 2017. PMID: 28437794 Review.
Cited by
-
A Real-World Analysis of the Population with Hepatitis C Virus Infection Affected by Type 2 Diabetes in Italy: Patients' Characteristics, Comorbidity Profiles and Treatment Patterns.Medicina (Kaunas). 2025 Mar 28;61(4):614. doi: 10.3390/medicina61040614. Medicina (Kaunas). 2025. PMID: 40282905 Free PMC article.
-
Real World Evidence on Hormone Receptor Positive and Human Epidermal Growth Factor Receptor 2 Negative Metastatic Breast Cancer in Italy: Insights From 2017 to 2021 Data.Clinicoecon Outcomes Res. 2025 Mar 6;17:147-155. doi: 10.2147/CEOR.S496606. eCollection 2025. Clinicoecon Outcomes Res. 2025. PMID: 40066280 Free PMC article.
References
-
- World Health Organization. Global health sector strategy on viral hepatitis 2016–21. Available from: https://apps.who.int/iris/handle/10665/246177. Accessed February 16, 2023.
LinkOut - more resources
Full Text Sources

