Review of optical reporters of radiation effects in vivo: tools to quantify improvements in radiation delivery technique
- PMID: 37560327
- PMCID: PMC10409499
- DOI: 10.1117/1.JBO.28.8.080901
Review of optical reporters of radiation effects in vivo: tools to quantify improvements in radiation delivery technique
Abstract
Significance: Radiation damage studies are used to optimize radiotherapy treatment techniques. Although biological indicators of damage are the best assays of effect, they are highly variable due to biological heterogeneity. The free radical radiochemistry can be assayed with optical reporters, allowing for high precision titration of techniques.
Aim: We examine the optical reporters of radiochemistry to highlight those with the best potential for translational use in vivo, as surrogates for biological damage assays, to inform on mechanisms.
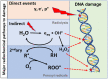
Approach: A survey of the radical chemistry effects from reactive oxygen species (ROS) and oxygen itself was completed to link to DNA or biological damage. Optical reporters of ROS include fluorescent, phosphorescent, and bioluminescent molecules that have a variety of activation pathways, and each was reviewed for its in vivo translation potential.
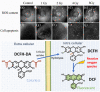
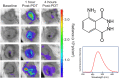
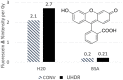
Results: There are molecular reporters of ROS having potential to report within living systems, including derivatives of luminol, 2'7'-dichlorofluorescein diacetate, Amplex Red, and fluorescein. None have unique specificity to singular ROS species. Macromolecular engineered reporters unique to specific ROS are emerging. The ability to directly measure oxygen via reporters, such as Oxyphor and protoporphyrin IX, is an opportunity to quantify the consumption of oxygen during ROS generation, and this translates from in vitro to in vivo use. Emerging techniques, such as ion particle beams, spatial fractionation, and ultra-high dose rate FLASH radiotherapy, provide the motivation for these studies.
Conclusions: In vivo optical reporters of radiochemistry are quantitatively useful for comparing radiotherapy techniques, although their use comes at the cost of the unknown connection to the mechanisms of radiobiological damage. Still their lower measurement uncertainty, compared with biological response assay, makes them an invaluable tool. Linkage to DNA damage and biological damage is needed, and measures such as oxygen consumption serve as useful surrogate measures that translate to in vivo use.
Keywords: fluorescence; phosphorescence; radiation; radiotherapy; reactive oxygen species.
© 2023 The Authors.
Figures







Similar articles
-
Radiotherapy Using High-Intensity Pulsed Radiation Beams (FLASH): A Radiation-Chemical Perspective.Radiat Res. 2020 Dec 1;194(6):607-617. doi: 10.1667/RADE-19-00016. Radiat Res. 2020. PMID: 33348369
-
Radiation chemistry comes before radiation biology.Int J Radiat Biol. 2009 Jan;85(1):9-25. doi: 10.1080/09553000802640401. Int J Radiat Biol. 2009. PMID: 19205982 Review.
-
The importance of radiation chemistry to radiation and free radical biology (The 2008 Silvanus Thompson Memorial Lecture).Br J Radiol. 2009 Feb;82(974):89-104. doi: 10.1259/bjr/60186130. Br J Radiol. 2009. PMID: 19168690 Review.
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Evaluation of assays for the measurement of bovine neutrophil reactive oxygen species.Vet Immunol Immunopathol. 2007 Jan 15;115(1-2):107-25. doi: 10.1016/j.vetimm.2006.09.009. Epub 2006 Sep 26. Vet Immunol Immunopathol. 2007. PMID: 17067684

