Kartogenin-enhanced dynamic hydrogel ameliorates intervertebral disc degeneration via restoration of local redox homeostasis
- PMID: 37560412
- PMCID: PMC10407629
- DOI: 10.1016/j.jot.2023.07.002
Kartogenin-enhanced dynamic hydrogel ameliorates intervertebral disc degeneration via restoration of local redox homeostasis
Abstract
Introduction: Over-activation of oxidative stress due to impaired antioxidant functions in nucleus pulpous (NP) has been identified as a key factor contributing to intervertebral disc degeneration (IVDD). While Kartogenin (KGN) has previously demonstrated antioxidant properties on articular cartilage against osteoarthritis, its effects on NP degeneration have yet to be fully understood.
Objectives: This study aimed to investigate the protective effects of KGN on nucleus pulpous cells (NPCs) against an inflammatory environment induced by interleukin (IL)-1β, as well as to explore the therapeutic potential of KGN-enhanced dynamic hydrogel in preventing IVDD.
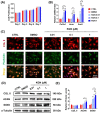
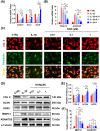
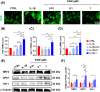
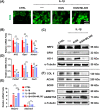
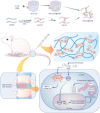
Methods: NPCs were isolated from rat caudal IVDs and subjected to treatment with KGN at varying concentrations (ranging from 0.01 to 1 μM) in the presence of IL-1β. The expression of extracellular matrix (ECM) anabolism markers was quantitatively assessed at both the mRNA and protein levels. Additionally, intracellular reactive oxygen species and antioxidant enzyme expression were evaluated, along with the role of nuclear factor erythroid 2-related factor 2 (NRF2). Based on these findings, a dynamic self-healing hydrogel loaded with KGN was developed through interconnecting networks. Subsequently, KGN-enhanced dynamic hydrogel was administered into rat caudal IVDs that had undergone puncture injury, followed by radiographic analysis and immunohistochemical staining to evaluate the therapeutic efficacy.
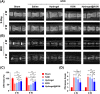
Results: In vitro treatments utilizing KGN were observed to maintain ECM synthesis and inhibit catabolic activities in IL-1β-stimulated NPCs. The mechanism behind this protective effect of KGN on NPCs was found to involve the asctivation of NRF2 and downstream antioxidant enzymes, including glutathione peroxidase 1 and heme oxygenase 1. This was further supported by the loss of both antioxidant and anabolic effects upon pharmacological inhibition of NRF2. Furthermore, a self-healing hydrogel was developed and loaded with KGN to achieve localized and sustained release of the compound. The injection of KGN-enhanced hydrogel effectively ameliorated the degradation of NP ECM and mitigated inflammation in a rat model of puncture-induced IVDD.
Conclusions: Our results indicate that KGN exhibits potential as a therapeutic agent for NP degeneration, and that KGN-enhanced dynamic hydrogel represents a novel approach for treating IVDD by restoring redox homeostasis in NP.The translational potential of this article: The dysregulation of oxidant and antioxidant balance has been shown to impede the repair and regeneration of NP, thereby hastening the progression of IVDD following injury. The present investigation has demonstrated that the sustained release of KGN promotes the synthesis of ECM in vitro and mitigates the progression of IVDD in vivo by restoring redox equilibrium, thereby presenting a novel therapeutic candidate based on the antioxidant properties of KGN for the treatment of IVDD.
Keywords: Dynamic hydrogel; Intervertebral disc degeneration; Kartogenin; NRF2; Nucleus pulposus.
© 2023 The Authors.
Conflict of interest statement
A conflict of interest occurs when an individual's objectivity is potentially compromised by a desire for financial gain, prominence, professional advancement or a successful outcome. The Editors of the Journal of Orthopaedic Translation strive to ensure that what is published in the Journal is as balanced, objective and evidence-based as possible. Since it can be difficult to distinguish between an actual conflict of interest and a perceived conflict of interest, the Journal requires authors to disclose all and any potential conflicts of interest.
Figures





References
-
- Armbrecht G., Felsenberg D., Ganswindt M., Lunt M., Kaptoge S.K., Abendroth K., et al. Degenerative inter-vertebral disc disease osteochondrosis intervertebralis in Europe: prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Rheumatology. 2017;56:1189–1199. - PMC - PubMed
-
- Berman B.M., Langevin H.M., Witt C.M., Dubner R. Acupuncture for chronic low back pain. N Engl J Med. 2010;363:454–461. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

