Integration of Cypoviruses into polyhedrin matrix
- PMID: 37560430
- PMCID: PMC10408579
- DOI: 10.1039/d3na00393k
Integration of Cypoviruses into polyhedrin matrix
Abstract
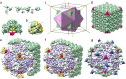
Unlike in other viruses, in Cypoviruses the genome is doubly protected since their icosahedral capsids are embedded into a perfect polyhedrin crystal. Current experimental methods cannot resolve the resulting interface structure and we propose a symmetry-based approach to predict it. We reveal a remarkable match between the surfaces of Cypovirus and the outer polyhedrin matrix. The match arises due to the preservation of the common tetragonal symmetry, allowing perfect contacts of polyhedrin trimers with VP1 and VP5 capsid proteins. We highlight a crucial role of the VP5 proteins in embedding the Cypovirus into the polyhedrin matrix and discuss the relationship between the nucleoside triphosphatase activity of the proteins and their role in the superstructure formation. Additionally, we propose an electrostatic mechanism that drives the viral superstructure disassembly occurring in the alkaline environment of the insect intestines. Our study may underpin novel strategies for engineering proteinaceous nanocontainers in diverse biotechnological and chemical applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Mori H. and Metcalf P., in Insect Virology, ed. S. Asgari and K. N. Johnson, Caister Academic Press, Poole, 2010, ch. 13, pp. 307–323
-
- Coulibaly F. Adv. Virus Res. 2019;105:275–335. - PubMed
LinkOut - more resources
Full Text Sources

