Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM)
- PMID: 37560967
- PMCID: PMC10561817
- DOI: 10.1681/ASN.0000000000000189
Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM)
Abstract
Significance statement: Mesenchymal stromal cells (MSCs) may offer a novel therapy for diabetic kidney disease (DKD), although clinical translation of this approach has been limited. The authors present findings from the first, lowest dose cohort of 16 adults with type 2 diabetes and progressive DKD participating in a randomized, placebo-controlled, dose-escalation phase 1b/2a trial of next-generation bone marrow-derived, anti-CD362 antibody-selected allogeneic MSCs (ORBCEL-M). A single intravenous (iv) infusion of 80×10 6 cells was safe and well-tolerated, with one quickly resolved infusion reaction in the placebo group and no subsequent treatment-related serious adverse events (SAEs). Compared with placebo, the median annual rate of decline in eGFR was significantly lower with ORBCEL-M, although mGFR did not differ. The results support further investigation of ORBCEL-M in this patient population in an appropriately sized phase 2b study.
Background: Systemic therapy with mesenchymal stromal cells may target maladaptive processes involved in diabetic kidney disease progression. However, clinical translation of this approach has been limited.
Methods: The Novel Stromal Cell Therapy for Diabetic Kidney Disease (NEPHSTROM) study, a randomized, placebo-controlled phase 1b/2a trial, assesses safety, tolerability, and preliminary efficacy of next-generation bone marrow-derived, anti-CD362-selected, allogeneic mesenchymal stromal cells (ORBCEL-M) in adults with type 2 diabetes and progressive diabetic kidney disease. This first, lowest dose cohort of 16 participants at three European sites was randomized (3:1) to receive intravenous infusion of ORBCEL-M (80×10 6 cells, n =12) or placebo ( n =4) and was followed for 18 months.
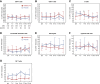
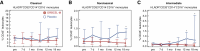
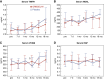
Results: At baseline, all participants were negative for anti-HLA antibodies and the measured GFR (mGFR) and estimated GFR were comparable between groups. The intervention was safe and well-tolerated. One placebo-treated participant had a quickly resolved infusion reaction (bronchospasm), with no subsequent treatment-related serious adverse events. Two ORBCEL-M recipients died during follow-up of causes deemed unrelated to the trial intervention; one recipient developed low-level anti-HLA antibodies. The median annual rate of kidney function decline after ORBCEL-M therapy compared with placebo did not differ by mGFR, but was significantly lower by eGFR estimated by the Chronic Kidney Disease Epidemiology Collaboration and Modification of Diet in Renal Disease equations. Immunologic profiling provided evidence of preservation of circulating regulatory T cells, lower natural killer T cells, and stabilization of inflammatory monocyte subsets in those receiving the cell therapy compared with placebo.
Conclusions: Findings indicate safety and tolerability of intravenous ORBCEL-M cell therapy in the trial's lowest dose cohort. The rate of decline in eGFR (but not mGFR) over 18 months was significantly lower among those receiving cell therapy compared with placebo. Further studies will be needed to determine the therapy's effect on CKD progression.
Clinical trial registration number: ClinicalTrial.gov NCT02585622 .
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
P. Cockwell reports Advisory or Leadership Role: UK Kidney Association (President and Trustee); Speakers Bureau: Amgen; and Other Interests or Relationships: Boehringer Ingleheim—non-remunerated research collaboration, AstraZeneca—non-remunerated clinical development program, and Glaxo Smith Kline—non-remunerated sponsored session chair at ISN 2020. S.J. Elliman is an employee of and equity holder in Orbsen Therapeutics. He was not involved in recruitment or follow-up of participants enrolled in the trial, nor was involved in the collection, analysis, and interpretation of the data presented herein. S.J. Elliman also reports Research Funding: Orbsen Therapeutics; Patents or Royalties: Orbsen Therapeutics; and Speakers Bureau: Orbsen Therapeutics. W.E. Fibbe reports Consultancy: Boost Pharma, Glycostem Therapeutics (iDMC), and Starfish Innovations; and Advisory or Leadership Role: Starfish Innovations. M.D. Griffin reports honoraria from the American Society of Nephrology, Hebei Medical University in China, Novo Nordisk, and Théa Pharma Ltd. in Ireland, as well as advisory roles as an Editorial Board member for the journals
Figures




Comment in
-
MSC therapy for diabetic kidney disease and nephrotic syndrome.Nat Rev Nephrol. 2023 Dec;19(12):754-755. doi: 10.1038/s41581-023-00776-z. Nat Rev Nephrol. 2023. PMID: 37783947 Free PMC article. No abstract available.
-
Cell Therapies in Diabetic Kidney Disease: Is It Time for Clinical Translation?J Am Soc Nephrol. 2023 Dec 1;34(12):2051-2052. doi: 10.1681/ASN.0000000000000230. J Am Soc Nephrol. 2023. PMID: 38039089 Free PMC article. No abstract available.
References
-
- Safiri S, Nejadghaderi SA, Karamzad N, Kaufman JS, Carson-Chahhoud K, Bragazzi NL. Global, regional and national burden of cancers attributable to high fasting plasma glucose in 204 countries and territories, 1990-2019. Front Endocrinol (Lausanne). 2022;13:879890. doi: 10.3389/fendo.2022.879890 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

