Long-Term Treatment With Tenofovir Alafenamide for Chronic Hepatitis B Results in High Rates of Viral Suppression and Favorable Renal and Bone Safety
- PMID: 37561058
- PMCID: PMC10903997
- DOI: 10.14309/ajg.0000000000002468
Long-Term Treatment With Tenofovir Alafenamide for Chronic Hepatitis B Results in High Rates of Viral Suppression and Favorable Renal and Bone Safety
Abstract
Introduction: The results from 2 phase 3 studies, through 2 years, in chronic hepatitis B infection showed tenofovir alafenamide (TAF) had similar efficacy to tenofovir disoproxil fumarate (TDF) with superior renal and bone safety. We report updated results through 5 years.
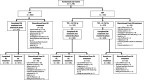
Methods: Patients with HBeAg-negative or HBeAg-positive chronic hepatitis B infection with or without compensated cirrhosis were randomized (2:1) to TAF 25 mg or TDF 300 mg once daily in double-blind (DB) fashion for up to 3 years, followed by open-label (OL) TAF up to 8 years. Efficacy (antiviral, biochemical, and serologic), resistance (deep sequencing of polymerase/reverse transcriptase and phenotyping), and safety, including renal and bone parameters, were evaluated by pooled analyses.
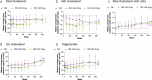
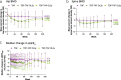
Results: Of 1,298 randomized and treated patients, 866 receiving TAF (DB and OL) and 432 receiving TDF with rollover to OL TAF at year 2 (n = 180; TDF→TAF3y) or year 3 (n = 202; TDF→TAF2y) were included. Fifty (4%) TDF patients who discontinued during DB were excluded. At year 5, 85%, 83%, and 90% achieved HBV DNA <29 IU/mL (missing = failure) in the TAF, TDF→TAF3y, and TDF→TAF2y groups, respectively; no patient developed TAF or TDF resistance. Median estimated glomerular filtration rate (by using Cockcroft-Gault) declined <2.5 mL/min, and mean declines of <1% in hip and spine bone mineral density were seen at year 5 in the TAF group; patients in the TDF→TAF groups had improvements in these parameters at year 5 after switching to OL TAF.
Discussion: Long-term TAF treatment resulted in high rates of viral suppression, no resistance, and favorable renal and bone safety.
Trial registration: ClinicalTrials.gov NCT01940341 NCT01940471.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



References
-
- World Health Organization. Hepatitis B Fact Sheet (https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b) (2021). Accessed September 2, 2021.
-
- Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol Hepatol 2018;3(6):383–403. - PubMed
-
- Seto WK, Lo YR, Pawlotsky JM, et al. Chronic hepatitis B virus infection. Lancet 2018;392(10161):2313–24. - PubMed
-
- Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015;121(20):3631–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

