Posttransfusion Sepsis Attributable to Bacterial Contamination in Platelet Collection Set Manufacturing Facility, United States
- PMID: 37561399
- PMCID: PMC10521617
- DOI: 10.3201/eid2910.230869
Posttransfusion Sepsis Attributable to Bacterial Contamination in Platelet Collection Set Manufacturing Facility, United States
Abstract
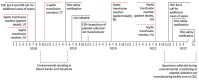
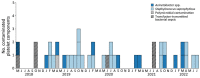
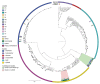
During May 2018‒December 2022, we reviewed transfusion-transmitted sepsis cases in the United States attributable to polymicrobial contaminated apheresis platelet components, including Acinetobacter calcoaceticus‒baumannii complex or Staphylococcus saprophyticus isolated from patients and components. Transfused platelet components underwent bacterial risk control strategies (primary culture, pathogen reduction or primary culture, and secondary rapid test) before transfusion. Environmental samples were collected from a platelet collection set manufacturing facility. Seven sepsis cases from 6 platelet donations from 6 different donors were identified in patients from 6 states; 3 patients died. Cultures identified Acinetobacter calcoaceticus‒baumannii complex in 6 patients and 6 transfused platelets, S. saprophyticus in 4 patients and 4 transfused platelets. Whole-genome sequencing showed environmental isolates from the manufacturer were closely related genetically to patient and platelet isolates, indicating the manufacturer was the most probable source of recurrent polymicrobial contamination. Clinicians should maintain awareness of possible transfusion-transmitted sepsis even when using bacterial risk control strategies.
Keywords: Acinetobacter calcoaceticus‒baumannii complex; Staphylococcus saprophyticus; United States; bacteria; bacterial contamination; blood safety; blood transfusions; manufacturing facility; platelet collection set; platelets; posttransfusion sepsis.
Figures


References
-
- Eder AF, Kennedy JM, Dy BA, Notari EP, Weiss JW, Fang CT, et al. ; American Red Cross Regional Blood Centers. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004-2006). Transfusion. 2007;47:1134–42. 10.1111/j.1537-2995.2007.01248.x - DOI - PubMed

