The variable conversion of neutralizing anti-SARS-CoV-2 single-chain antibodies to IgG provides insight into RBD epitope accessibility
- PMID: 37561410
- PMCID: PMC10505556
- DOI: 10.1093/protein/gzad008
The variable conversion of neutralizing anti-SARS-CoV-2 single-chain antibodies to IgG provides insight into RBD epitope accessibility
Erratum in
-
Correction to: The variable conversion of neutralizing anti-SARS-CoV-2 single-chain antibodies to IgG provides insight into RBD epitope accessibility.Protein Eng Des Sel. 2023 Jan 21;36:gzad018. doi: 10.1093/protein/gzad018. Protein Eng Des Sel. 2023. PMID: 37933088 Free PMC article. No abstract available.
Abstract
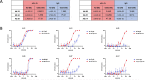
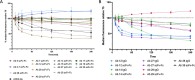
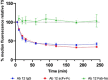
Monoclonal antibody (mAb) therapies have rapidly become a powerful class of therapeutics with applications covering a diverse range of clinical indications. Though most widely used for the treatment of cancer, mAbs are also playing an increasing role in the defense of viral infections, most recently with palivizumab for prevention and treatment of severe RSV infections in neonatal and pediatric populations. In addition, during the COVID-19 pandemic, mAbs provided a bridge to the rollout of vaccines; however, their continued role as a therapeutic option for those at greatest risk of severe disease has become limited due to the emergence of neutralization resistant Omicron variants. Although there are many techniques for the identification of mAbs, including single B cell cloning and immunization of genetically engineered mice, the low cost, rapid throughput and technological simplicity of antibody phage display has led to its widespread adoption in mAb discovery efforts. Here we used our 27-billion-member naïve single-chain antibody (scFv) phage library to identify a panel of neutralizing anti-SARS-CoV-2 scFvs targeting diverse epitopes on the receptor binding domain (RBD). Although typically a routine process, we found that upon conversion to IgG, a number of our most potent clones failed to maintain their neutralization potency. Kinetic measurements confirmed similar affinity to the RBD; however, mechanistic studies provide evidence that the loss of neutralization is a result of structural limitations likely arising from initial choice of panning antigen. Thus this work highlights a risk of scFv-phage panning to mAb conversion and the importance of initial antigen selection.
Keywords: SARS-CoV-2; antibody discovery; neutralizing antibodies; phage display; phage panning.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures



Similar articles
-
Novel human neutralizing mAbs specific for Spike-RBD of SARS-CoV-2.Sci Rep. 2021 May 26;11(1):11046. doi: 10.1038/s41598-021-90348-7. Sci Rep. 2021. PMID: 34040046 Free PMC article.
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Generation and utility of a single-chain fragment variable monoclonal antibody platform against a baculovirus expressed recombinant receptor binding domain of SARS-CoV-2 spike protein.Mol Immunol. 2022 Jan;141:287-296. doi: 10.1016/j.molimm.2021.12.006. Epub 2021 Dec 10. Mol Immunol. 2022. PMID: 34915268 Free PMC article.
-
Isolation and Characterization of Mouse Monoclonal Antibodies That Neutralize SARS-CoV-2 and Its Variants of Concern Alpha, Beta, Gamma and Delta by Binding Conformational Epitopes of Glycosylated RBD With High Potency.Front Immunol. 2021 Oct 26;12:750386. doi: 10.3389/fimmu.2021.750386. eCollection 2021. Front Immunol. 2021. PMID: 34764961 Free PMC article.
-
Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies.Biomed Pharmacother. 2020 Oct;130:110559. doi: 10.1016/j.biopha.2020.110559. Epub 2020 Aug 1. Biomed Pharmacother. 2020. PMID: 32768882 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

