Nucleus accumbens D1/D2 circuits control opioid withdrawal symptoms in mice
- PMID: 37561576
- PMCID: PMC10503809
- DOI: 10.1172/JCI163266
Nucleus accumbens D1/D2 circuits control opioid withdrawal symptoms in mice
Abstract
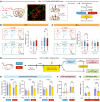
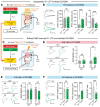
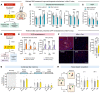
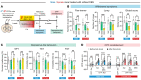
The nucleus accumbens (NAc) is the most promising target for drug use disorder treatment. Deep brain stimulation (DBS) of NAc is effective for drug use disorder treatment. However, the mechanisms by which DBS produces its therapeutic effects remain enigmatic. Here, we define a behavioral cutoff criterion to distinguish depressive-like behaviors and non-depressive-like behaviors in mice after morphine withdrawal. We identified a basolateral amygdala (BLA) to NAc D1 medium spiny neuron (MSN) pathway that controls depressive-like behaviors after morphine withdrawal. Furthermore, the paraventricular nucleus of thalamus (PVT) to NAc D2 MSN pathway controls naloxone-induced acute withdrawal symptoms. Optogenetically induced long-term potentiation with κ-opioid receptor (KOR) antagonism enhanced BLA to NAc D1 MSN signaling and also altered the excitation/inhibition balance of NAc D2 MSN signaling. We also verified that a new 50 Hz DBS protocol reversed morphine withdrawal-evoked abnormal plasticity in NAc. Importantly, this refined DBS treatment effectively alleviated naloxone-induced withdrawal symptoms and depressive-like behaviors and prevented stress-induced reinstatement. Taken together, the results demonstrated that input- and cell type-specific synaptic plasticity underlies morphine withdrawal, which may lead to novel targets for the treatment of opioid use disorder.
Keywords: Addiction; Depression; Mouse models; Neuroscience.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

