Nonviral base editing of KCNJ13 mutation preserves vision in a model of inherited retinal channelopathy
- PMID: 37561581
- PMCID: PMC10541187
- DOI: 10.1172/JCI171356
Nonviral base editing of KCNJ13 mutation preserves vision in a model of inherited retinal channelopathy
Abstract
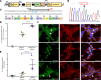
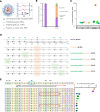
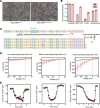
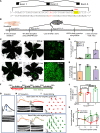
Clinical genome editing is emerging for rare disease treatment, but one of the major limitations is the targeting of CRISPR editors' delivery. We delivered base editors to the retinal pigmented epithelium (RPE) in the mouse eye using silica nanocapsules (SNCs) as a treatment for retinal degeneration. Leber congenital amaurosis type 16 (LCA16) is a rare pediatric blindness caused by point mutations in the KCNJ13 gene, a loss of function inwardly rectifying potassium channel (Kir7.1) in the RPE. SNCs carrying adenine base editor 8e (ABE8e) mRNA and sgRNA precisely and efficiently corrected the KCNJ13W53X/W53X mutation. Editing in both patient fibroblasts (47%) and human induced pluripotent stem cell-derived RPE (LCA16-iPSC-RPE) (17%) showed minimal off-target editing. We detected functional Kir7.1 channels in the edited LCA16-iPSC-RPE. In the LCA16 mouse model (Kcnj13W53X/+ΔR), RPE cells targeted SNC delivery of ABE8e mRNA preserved normal vision, measured by full-field electroretinogram (ERG). Moreover, multifocal ERG confirmed the topographic measure of electrical activity primarily originating from the edited retinal area at the injection site. Preserved retina structure after treatment was established by optical coherence tomography (OCT). This preclinical validation of targeted ion channel functional rescue, a challenge for pharmacological and genomic interventions, reinforced the effectiveness of nonviral genome-editing therapy for rare inherited disorders.
Keywords: Gene therapy; Ion channels; Nanotechnology; Ophthalmology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

