Clinical-genomic determinants of immune checkpoint blockade response in head and neck squamous cell carcinoma
- PMID: 37561583
- PMCID: PMC10541199
- DOI: 10.1172/JCI169823
Clinical-genomic determinants of immune checkpoint blockade response in head and neck squamous cell carcinoma
Abstract
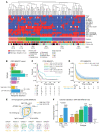
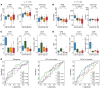
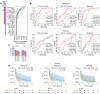
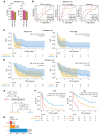
BACKGROUNDRecurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) is generally an incurable disease, with patients experiencing median survival of under 10 months and significant morbidity. While immune checkpoint blockade (ICB) drugs are effective in approximately 20% of patients, the remaining experience limited clinical benefit and are exposed to potential adverse effects and financial costs. Clinically approved biomarkers, such as tumor mutational burden (TMB), have a modest predictive value in HNSCC.METHODSWe analyzed clinical and genomic features, generated using whole-exome sequencing, in 133 ICB-treated patients with R/M HNSCC, of whom 69 had virus-associated and 64 had non-virus-associated tumors.RESULTSHierarchical clustering of genomic data revealed 6 molecular subtypes characterized by a wide range of objective response rates and survival after ICB therapy. The prognostic importance of these 6 subtypes was validated in an external cohort. A random forest-based predictive model, using several clinical and genomic features, predicted progression-free survival (PFS), overall survival (OS), and response with greater accuracy than did a model based on TMB alone. Recursive partitioning analysis identified 3 features (systemic inflammatory response index, TMB, and smoking signature) that classified patients into risk groups with accurate discrimination of PFS and OS.CONCLUSIONThese findings shed light on the immunogenomic characteristics of HNSCC tumors that drive differential responses to ICB and identify a clinical-genomic classifier that outperformed the current clinically approved biomarker of TMB. This validated predictive tool may help with clinical risk stratification in patients with R/M HNSCC for whom ICB is being considered.FUNDINGFundación Alfonso Martín Escudero, NIH R01 DE027738, US Department of Defense CA210784, The Geoffrey Beene Cancer Research Center, The MSKCC Population Science Research Program, the Jayme Flowers Fund, the Sebastian Nativo Fund, and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Keywords: Cancer immunotherapy; Head and neck cancer; Immunology; Oncology.
Figures


References
-
- Ferlay J, et al. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. Cancer Incidence and Mortality Worldwide; 2013.

