Vascular smooth muscle-specific YAP/TAZ deletion triggers aneurysm development in mouse aorta
- PMID: 37561588
- PMCID: PMC10544211
- DOI: 10.1172/jci.insight.170845
Vascular smooth muscle-specific YAP/TAZ deletion triggers aneurysm development in mouse aorta
Abstract
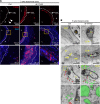
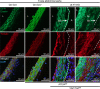
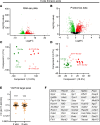
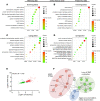
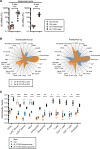
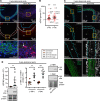
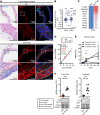
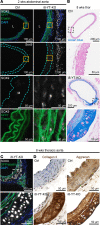
Inadequate adaption to mechanical forces, including blood pressure, contributes to development of arterial aneurysms. Recent studies have pointed to a mechanoprotective role of YAP and TAZ in vascular smooth muscle cells (SMCs). Here, we identified reduced expression of YAP1 in human aortic aneurysms. Vascular SMC-specific knockouts (KOs) of YAP/TAZ were thus generated using the integrin α8-Cre (Itga8-Cre) mouse model (i8-YT-KO). i8-YT-KO mice spontaneously developed aneurysms in the abdominal aorta within 2 weeks of KO induction and in smaller arteries at later times. The vascular specificity of Itga8-Cre circumvented gastrointestinal effects. Aortic aneurysms were characterized by elastin disarray, SMC apoptosis, and accumulation of proteoglycans and immune cell populations. RNA sequencing, proteomics, and myography demonstrated decreased contractile differentiation of SMCs and impaired vascular contractility. This associated with partial loss of myocardin expression, reduced blood pressure, and edema. Mediators in the inflammatory cGAS/STING pathway were increased. A sizeable increase in SOX9, along with several direct target genes, including aggrecan (Acan), contributed to proteoglycan accumulation. This was the earliest detectable change, occurring 3 days after KO induction and before the proinflammatory transition. In conclusion, Itga8-Cre deletion of YAP and TAZ represents a rapid and spontaneous aneurysm model that recapitulates features of human abdominal aortic aneurysms.
Keywords: Cardiovascular disease; Cell Biology; Hypertension; Vascular Biology; Vasculitis.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

