A phase I study of autologous mesenchymal stromal cells for severe steroid-dependent nephrotic syndrome
- PMID: 37561590
- PMCID: PMC10561718
- DOI: 10.1172/jci.insight.169424
A phase I study of autologous mesenchymal stromal cells for severe steroid-dependent nephrotic syndrome
Abstract
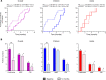
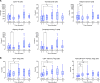
BACKGROUNDSevere forms of idiopathic nephrotic syndrome (INS) require prolonged immunosuppressive therapies and repeated courses of high-dose glucocorticoids. Mesenchymal stromal cells (MSCs) have promising immunomodulatory properties that may be employed therapeutically to reduce patient exposure to medications and their side effects.METHODSWe performed a phase I open-label trial assessing safety and feasibility of autologous bone marrow-derived MSCs (BM-MSCs) in children and young adults with severe forms of steroid-dependent nephrotic syndrome. Following autologous BM-MSC preparation and infusion, oral immunosuppression was tapered. Safety, efficacy, and immunomodulatory effects in vivo were monitored for 12 months.RESULTSSixteen patients (10 children, 6 adults) were treated. Adverse events were limited and not related to BM-MSC infusions. All patients relapsed during follow-up, but in the 10 treated children, time to first relapse was delayed (P = 0.02) and number of relapses was reduced (P = 0.002) after BM-MSC infusion, compared with the previous 12 months. Cumulative prednisone dose was also reduced at 12 months compared with baseline (P < 0.05). No treatment benefit was observed in adults.In children, despite tapering of immunosuppression, clinical benefit was mirrored by a significant reduction in total CD19+, mature, and memory B cells and an increase in regulatory T cells in vivo up to 3-6 months following BM-MSC infusionCONCLUSIONTreatment with autologous BM-MSCs is feasible and safely reduces relapses and immunosuppression at 12 months in children with severe steroid-dependent INS. Immunomodulatory studies suggest that repeating MSC infusions at 3-6 months may sustain benefit.TRIAL REGISTRATIONEudraCT 2016-004804-77.FUNDINGAIFA Ricerca Indipendente 2016-02364623.
Keywords: Clinical Trials; Immunotherapy; Nephrology.
Figures


Comment in
-
MSC therapy for diabetic kidney disease and nephrotic syndrome.Nat Rev Nephrol. 2023 Dec;19(12):754-755. doi: 10.1038/s41581-023-00776-z. Nat Rev Nephrol. 2023. PMID: 37783947 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

