Pathogenic myelin-specific antibodies in multiple sclerosis target conformational proteolipid protein 1-anchored membrane domains
- PMID: 37561592
- PMCID: PMC10541191
- DOI: 10.1172/JCI162731
Pathogenic myelin-specific antibodies in multiple sclerosis target conformational proteolipid protein 1-anchored membrane domains
Abstract
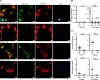
B cell clonal expansion and cerebrospinal fluid (CSF) oligoclonal IgG bands are established features of the immune response in multiple sclerosis (MS). Clone-specific recombinant monoclonal IgG1 Abs (rAbs) derived from MS patient CSF plasmablasts bound to conformational proteolipid protein 1 (PLP1) membrane complexes and, when injected into mouse brain with human complement, recapitulated histologic features of MS pathology: oligodendrocyte cell loss, complement deposition, and CD68+ phagocyte infiltration. Conformational PLP1 membrane epitopes were complex and governed by the local cholesterol and glycolipid microenvironment. Abs against conformational PLP1 membrane complexes targeted multiple surface epitopes, were enriched within the CSF compartment, and were detected in most MS patients, but not in inflammatory and noninflammatory neurologic controls. CSF PLP1 complex Abs provide a pathogenic autoantibody biomarker specific for MS.
Keywords: Antigen; Autoimmunity; Immunoglobulins; Multiple sclerosis; Neuroscience.
Figures








References
-
- Van Sande M, et al. [Further findings in the electrophoretic study of cerebrospinal fluid proteins in multiple sclerosis] Acta Neurol Psychiatr Belg. 1959;59:762–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

