Rescue of Alzheimer's disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells
- PMID: 37561625
- PMCID: PMC10617121
- DOI: 10.1016/j.celrep.2023.112956
Rescue of Alzheimer's disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells
Abstract
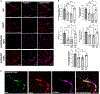
Alzheimer's disease (AD) is the most prevalent cause of dementia; microglia have been implicated in AD pathogenesis, but their role is still matter of debate. Our study showed that single systemic wild-type (WT) hematopoietic stem and progenitor cell (HSPC) transplantation rescued the AD phenotype in 5xFAD mice and that transplantation may prevent microglia activation. Indeed, complete prevention of memory loss and neurocognitive impairment and decrease of β-amyloid plaques in the hippocampus and cortex were observed in the WT HSPC-transplanted 5xFAD mice compared with untreated 5xFAD mice and with mice transplanted with 5xFAD HSPCs. Neuroinflammation was also significantly reduced. Transcriptomic analysis revealed a significant decrease in gene expression related to "disease-associated microglia" in the cortex and "neurodegeneration-associated endothelial cells" in the hippocampus of the WT HSPC-transplanted 5xFAD mice compared with diseased controls. This work shows that HSPC transplant has the potential to prevent AD-associated complications and represents a promising therapeutic avenue for this disease.
Keywords: Alzheimer's disease; CP: Neuroscience; CP: Stem cell research; hematopoietic stem and and progenitor cells; microgliosis; neuroinflammation.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.C. is a co-founder, shareholder, and member of both the scientific board and board of directors of Papillon Therapeutics, Inc. S.C. serves as a consultant for AVROBIO and receives compensation for these services. S.C. also serves as a member of the scientific review board and the board of trustees of Cystinosis Research Foundation. This work is covered in the patent entitled “Methods for treating Alzheimer’s Disease” (#114198-8190). The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
Figures

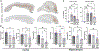


References
-
- Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier WM, Kern S, Ousset PJ, Maruff P, Skoog I, et al. (2019). Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 15, 888–898. 10.1016/j.jalz.2019.04.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

