Self-application of aminoglycoside-based creams to treat cutaneous leishmaniasis in travelers
- PMID: 37561802
- PMCID: PMC10443860
- DOI: 10.1371/journal.pntd.0011492
Self-application of aminoglycoside-based creams to treat cutaneous leishmaniasis in travelers
Abstract
Background: In endemic foci, the use of an aquaphilic cream containing paromomycin with/without gentamicin to treat cutaneous leishmaniasis (CL) is safe, painless and cures 78-82% of patients with New and Old World CL. Self-application in travelers requires evaluation.
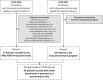
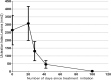
Methods: Travelers with 1-10 lesions of confirmed CL were prospectively treated with the paromomycin-gentamicin formulation (WR279396, 2012-2017, Group 1) and carefully follow up, or treated with a locally produced paromomycin-only cream (2018-2022, Group 2). The cream was applied once under supervision, then self-applied daily for 20-30 days. A cured lesion was defined as 100% re-epithelialization at day 42 without relapse at three months.
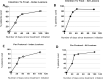
Results: Medical features were similar in Group 1 (17 patients), and Group 2 (23 patients). Patients were infected with either Leishmania major, L. infantum, L. killicki, L. guyanensis, L. braziliensis, or L. naiffi. Intention-to-treat and per-protocol cure rates were 82% (95% confidence interval (CI) [64.23;100.00]) and 87% (95% CI [71,29;100.00]) in Group 1, and 69% (95% CI [50.76; 88.37]) and 76% (95% CI [57.97; 94.41]) in Group 2. In the pooled Group 1&2, 75% (95% CI [61.58;88.42]) (30/40) and 81% (95% CI [68,46;93.6]) (30/37) of patients were cured in intention-to-treat and per-protocol, respectively. There were no significant differences observed in the success rates between Old World and New World CL (83.3% vs. 60%, p = 0.14). Prospective observations in Group 1 showed that adverse events were mainly pruritus (24%) and pain (18%) on lesions (all mild or moderate). No mucosal involvement was observed in either group.
Discussion: In this representative population of travelers who acquired CL either in the Old or New World, the 81% per-protocol cure rate of a self-applied aminoglycoside cream was similar to that observed in clinical trials.
Copyright: © 2023 Mouri et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests : PB is member of the scientific advisory committee of Drugs for Neglected Diseases initiative.
Figures

References
-
- Burza S, Croft SL, Boelaert M. Leishmaniasis. The Lancet. 2018. Sep 15;392(10151):951–70. - PubMed
-
- Masmoudi A, Maalej N, Mseddi M, Souissi A, Turki H, Boudaya S, et al. Glucantime par voie parentérale: bénéfice versus toxicité. Médecine Mal Infect [Internet]. 2005. Jan [cited 2023 Feb 9];35(1):42–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0399077X04002896. - PubMed
-
- Ribeiro AL, Drummond JB, Volpini AC, Andrade AC, Passos VM. Electrocardiographic changes during low-dose, short-term therapy of cutaneous leishmaniasis with the pentavalent antimonial meglumine. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. 1999. Mar;32(3):297–301. doi: 10.1590/s0100-879x1999000300008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

