Genotype, oxidase status, and preceding infection or autoinflammation do not affect allogeneic HCT outcomes for CGD
- PMID: 37562003
- PMCID: PMC10862239
- DOI: 10.1182/blood.2022019586
Genotype, oxidase status, and preceding infection or autoinflammation do not affect allogeneic HCT outcomes for CGD
Abstract
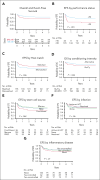
Chronic granulomatous disease (CGD) is a primary immunodeficiency characterized by life-threatening infections and inflammatory conditions. Hematopoietic cell transplantation (HCT) is the definitive treatment for CGD, but questions remain regarding patient selection and impact of active disease on transplant outcomes. We performed a multi-institutional retrospective and prospective study of 391 patients with CGD treated either conventionally (non-HCT) enrolled from 2004 to 2018 or with HCT from 1996 to 2018. Median follow-up after HCT was 3.7 years with a 3-year overall survival of 82% and event-free survival of 69%. In a multivariate analysis, a Lansky/Karnofsky score <90 and use of HLA-mismatched donors negatively affected survival. Age, genotype, and oxidase status did not affect outcomes. Before HCT, patients had higher infection density, higher frequency of noninfectious lung and liver diseases, and more steroid use than conventionally treated patients; however, these issues did not adversely affect HCT survival. Presence of pre-HCT inflammatory conditions was associated with chronic graft-versus-host disease. Graft failure or receipt of a second HCT occurred in 17.6% of the patients and was associated with melphalan-based conditioning and/or early mixed chimerism. At 3 to 5 years after HCT, patients had improved growth and nutrition, resolved infections and inflammatory disease, and lower rates of antimicrobial prophylaxis or corticosteroid use compared with both their baseline and those of conventionally treated patients. HCT leads to durable resolution of CGD symptoms and lowers the burden of the disease. Patients with active infection or inflammation are candidates for transplants; HCT should be considered before the development of comorbidities that could affect performance status. This trial was registered at www.clinicaltrials.gov as #NCT02082353.
Conflict of interest statement
Conflict-of-interest disclosure: J.W.L. is an employee and shareholder of bluebird bio; a speaker and advisory board member of Horizon Therapeutics and Sobi; and a consultant for Prime Medicine. R.A.M. serves as a section editor for UpToDate and is an advisory board member of Horizon Therapeutics and Sobi. J.R.H. receives research support from Regeneron, CSL-Behring, ADMA, Enzyvant and author royalties from UpToDate. L.M.B. has received clinical trial support from Medac GmbH; is a member of the data safety monitoring board for a clinical trial with Jasper Therapeutics; and has served on an advisory board for Horizon Therapeutics. J.J.B. is an advisory board member of Horizon Therapeutics and Sobi. G.D.E.C. is a consultant for Miltenyi Biotec. L.R.F.S. serves as a consultant for Takeda, Grifols, and ADMA and as an advisory board member for Horizon, CSL-Behring, Enzyvant, and Incyte. S.E.P. receives support for the conduct of sponsored trials through Boston Children’s Hospital from AlloVir, Atara, and Jasper Therapeutics; is a consultant for CellEvolve, Smart Immune, and Regeneron; and has intellectual property related to the development of off-the-shelf viral-specific cytotoxic T lymphocytes, with all rights assigned to Memorial Sloan Kettering Cancer Center. C.C.D. is a consultant for Alexion and Jazz Pharmaceuticals. E.H. is an advisory board member of Octapharma, Takeda, Jasper, Therapeutics, and Rocket Pharma. M.A.P. is an advisory board member for Novartis, Gentibio, bluebird bio, Vertex, Medexus, and Equillium; and has received study support from Miltenyi Biotec and Adaptive. J.M.P. receives royalties from UpToDate and their spouse is an employee of Invitae. H.L.M. is part of a cooperative agreement between NIAID and Prime Medicine. The remaining authors declare no competing financial interests.
Figures




Comment in
-
HCT alleviates disease burden in CGD.Blood. 2023 Dec 14;142(24):2043-2045. doi: 10.1182/blood.2023021538. Blood. 2023. PMID: 38095925 No abstract available.
References
-
- Leiding JW, Holland SM. In: GeneReviews(®) [Internet] Adam MP, Feldman J, Mirzaa GM, et al., editors. University of Washington, Seattle; Seattle, WA: 1993-2023. Published 9 August 2012. Updated 21 April 2022. Chronic granulomatous disease. - PubMed
-
- Winkelstein JA, Marino MC, Johnston RB, Jr., et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79(3):155–169. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U01 TR001263/TR/NCATS NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U54 AI082973/AI/NIAID NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- KL2 TR002492/TR/NCATS NIH HHS/United States
- U01 HL069294/HL/NHLBI NIH HHS/United States
- U54 NS064808/NS/NINDS NIH HHS/United States
- ZIA AI001222/ImNIH/Intramural NIH HHS/United States
- Z01 AI000644/ImNIH/Intramural NIH HHS/United States
- U10 HL069254/HL/NHLBI NIH HHS/United States
- R13 AI094943/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

