Parenchymal border macrophages regulate tau pathology and tau-mediated neurodegeneration
- PMID: 37562846
- PMCID: PMC10415611
- DOI: 10.26508/lsa.202302087
Parenchymal border macrophages regulate tau pathology and tau-mediated neurodegeneration
Abstract
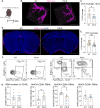
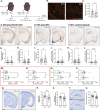
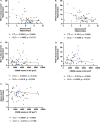
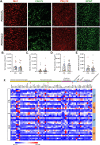
Parenchymal border macrophages (PBMs) reside close to the central nervous system parenchyma and regulate CSF flow dynamics. We recently demonstrated that PBMs provide a clearance pathway for amyloid-β peptide, which accumulates in the brain in Alzheimer's disease (AD). Given the emerging role for PBMs in AD, we explored how tau pathology affects the CSF flow and the PBM populations in the PS19 mouse model of tau pathology. We demonstrated a reduction of CSF flow, and an increase in an MHCII+PBM subpopulation in PS19 mice compared with WT littermates. Consequently, we asked whether PBM dysfunction could exacerbate tau pathology and tau-mediated neurodegeneration. Pharmacological depletion of PBMs in PS19 mice led to an increase in tau pathology and tau-dependent neurodegeneration, which was independent of gliosis or aquaporin-4 depolarization, essential for the CSF-ISF exchange. Together, our results identify PBMs as novel cellular regulators of tau pathology and tau-mediated neurodegeneration.
© 2023 Drieu et al.
Conflict of interest statement
DM Holtzman is listed as an inventor on a provisional patent from Washington University on TREM2 antibodies. DM Holtzman is listed as inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies. DM Holtzman co-founded and is on the scientific advisory board of C2N Diagnostics. DM Holtzman is on the scientific advisory board of Denali, Genentech, and Cajal Neurosciences and consults for Asteroid Therapeutics.
Figures


References
-
- Abe Y, Shimada M, Takeda Y, Enoki T, Omachi K, Abe S, Murakami M, Tanaka T, Goda W, Goto M, et al. (2020) Evaluation of patient positioning during digital tomosynthesis and reconstruction algorithms for ilizarov frames: A phantom study. Strategies Trauma Limb Reconstr 15: 1–6. 10.5005/jp-journals-10080-1446 - DOI - PMC - PubMed
-
- Ceyzériat K, Ben Haim L, Denizot A, Pommier D, Matos M, Guillemaud O, Palomares MA, Abjean L, Petit F, Gipchtein P, et al. (2018) Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer's disease. Acta Neuropathol Commun 6: 104. 10.1186/s40478-018-0606-1 - DOI - PMC - PubMed
-
- Da Mesquita S, Herz J, Wall M, Dykstra T, Alves de Lima K, Norris GT, Dabhi N, Kennedy T, Baker W, Kipnis J (2021. a) Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci Adv 7: eabe4601. 10.1126/sciadv.abe4601 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
