EBNA1 Inhibitors Block Proliferation of Spontaneous Lymphoblastoid Cell Lines From Patients With Multiple Sclerosis and Healthy Controls
- PMID: 37562974
- PMCID: PMC10414776
- DOI: 10.1212/NXI.0000000000200149
EBNA1 Inhibitors Block Proliferation of Spontaneous Lymphoblastoid Cell Lines From Patients With Multiple Sclerosis and Healthy Controls
Erratum in
-
Missing Full Disclosures.Neurol Neuroimmunol Neuroinflamm. 2025 Jan;12(1):e200342. doi: 10.1212/NXI.0000000000200342. Epub 2024 Oct 30. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39475708 Free PMC article. No abstract available.
Abstract
Background and objectives: Epstein-Barr virus (EBV) is a ubiquitous herpesvirus that establishes lifelong latency in memory B cells and has been identified as a major risk factor of multiple sclerosis (MS). B cell depletion therapies have disease-modifying benefit in MS. However, it is unclear whether this benefit is partly attributable to the elimination of EBV+ B cells. Currently, there are no EBV-specific antiviral therapies available for targeting EBV latent infection in MS and limited experimental models to study EBV in MS.
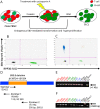
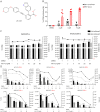
Methods: In this study, we describe the establishment of spontaneous lymphoblastoid cell lines (SLCLs) generated ex vivo with the endogenous EBV of patients with MS and controls and treated with either an Epstein-Barr virus nuclear antigen 1 (EBNA1) inhibitor (VK-1727) or cladribine, a nucleoside analog that eliminates B cells.
Results: We showed that a small molecule inhibitor of EBNA1, a critical regulator of the EBV life cycle, blocks the proliferation and metabolic activity of these SLCLs. In contrast to cladribine, a highly cytotoxic B cell depleting therapy currently used in MS, the EBNA1 inhibitor VK-1727 was cytostatic rather than cytotoxic and selective for EBV+ cells, while having no discernible effects on EBV- cells. We validate that VK-1727 reduces EBNA1 DNA binding at known viral and cellular sites by ChIP-qPCR.
Discussion: This study shows that patient-derived SLCLs provide a useful tool for interrogating the role of EBV+ B cells in MS and suggests that a clinical trial testing the effect of EBNA1 inhibitors in MS may be warranted.
Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a “work of the United States Government” for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
Conflict of interest statement
The Wistar Institute, on behalf of the authors T.E.M. and P.M.L., has filed patents covering composition of matter and their use on the small molecule disclosed here for the treatment of human cancer and other diseases (patent number WO2015073864, “EBNA1 Inhibitors and Their Method of Use”; WO2016183534, “EBNA1 Inhibitors and Methods using Same”). P.M.L. has an ownership interest in Vironika, LLC. Go to
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
