Effector-dependent structural transformation of a crystalline framework with allosteric effects on molecular recognition ability
- PMID: 37563107
- PMCID: PMC10415384
- DOI: 10.1038/s41467-023-40091-6
Effector-dependent structural transformation of a crystalline framework with allosteric effects on molecular recognition ability
Abstract
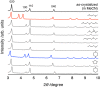
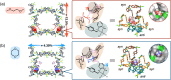
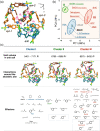
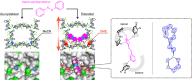
Structurally flexible porous crystals that combine high regularity and stimuli responsiveness have received attracted attention in connection with natural allostery found in regulatory systems of activity and function in biological systems. Porous crystals with molecular recognition sites in the inner pores are particularly promising for achieving elaborate functional control, where the local binding of effectors triggers their distortion to propagate throughout the structure. Here we report that the structure of a porous molecular crystal can be allosterically controlled by local adsorption of effectors within low-symmetry nanochannels with multiple molecular recognition sites. The exchange of effectors at the allosteric site triggers diverse conversion of the framework structure in an effector-dependent manner. In conjunction with the structural conversion, it is also possible to switch the molecular affinity at different recognition sites. These results may provide a guideline for the development of supramolecular materials with flexible and highly-ordered three-dimensional structures for biological applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Novel Porous Crystals with Macrocycle-Based Well-Defined Molecular Recognition Sites.Acc Chem Res. 2020 Mar 17;53(3):632-643. doi: 10.1021/acs.accounts.9b00566. Epub 2020 Jan 23. Acc Chem Res. 2020. PMID: 31970991 Review.
-
Structurally distributed surface sites tune allosteric regulation.Elife. 2021 Jun 16;10:e68346. doi: 10.7554/eLife.68346. Elife. 2021. PMID: 34132193 Free PMC article.
-
Correlating allostery with rigidity.Mol Biosyst. 2011 Feb;7(2):464-71. doi: 10.1039/c0mb00054j. Epub 2010 Nov 8. Mol Biosyst. 2011. PMID: 21060909
-
Crystal structure of Escherichia coli pyruvate kinase type I: molecular basis of the allosteric transition.Structure. 1995 Jul 15;3(7):729-41. doi: 10.1016/s0969-2126(01)00207-6. Structure. 1995. PMID: 8591049
-
Allosteric Regulation of Protein Kinases Downstream of PI3-Kinase Signalling.Adv Exp Med Biol. 2019;1163:279-311. doi: 10.1007/978-981-13-8719-7_12. Adv Exp Med Biol. 2019. PMID: 31707708 Review.
Cited by
-
Porous Supramolecular Crystalline Probe that Detects Non-Covalent Interactions Involved in Molecular Recognition of Furanic Compounds.Small. 2024 Dec;20(49):e2405507. doi: 10.1002/smll.202405507. Epub 2024 Jul 30. Small. 2024. PMID: 39076053 Free PMC article.
-
Materials Nanoarchitectonics at Dynamic Interfaces: Structure Formation and Functional Manipulation.Materials (Basel). 2024 Jan 4;17(1):271. doi: 10.3390/ma17010271. Materials (Basel). 2024. PMID: 38204123 Free PMC article. Review.
-
LABind: identifying protein binding ligand-aware sites via learning interactions between ligand and protein.Nat Commun. 2025 Aug 19;16(1):7712. doi: 10.1038/s41467-025-62899-0. Nat Commun. 2025. PMID: 40830361 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

