Norepinephrine release in the cerebellum contributes to aversive learning
- PMID: 37563141
- PMCID: PMC10415399
- DOI: 10.1038/s41467-023-40548-8
Norepinephrine release in the cerebellum contributes to aversive learning
Abstract
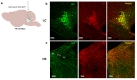
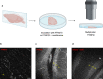
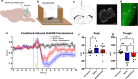
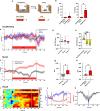
The modulation of dopamine release from midbrain projections to the striatum has long been demonstrated in reward-based learning, but the synaptic basis of aversive learning is far less characterized. The cerebellum receives axonal projections from the locus coeruleus, and norepinephrine release is implicated in states of arousal and stress, but whether aversive learning relies on plastic changes in norepinephrine release in the cerebellum is unknown. Here we report that in mice, norepinephrine is released in the cerebellum following an unpredicted noxious event (a foot-shock) and that this norepinephrine release is potentiated powerfully with fear acquisition as animals learn that a previously neutral stimulus (tone) predicts the aversive event. Importantly, both chemogenetic and optogenetic inhibition of the locus coeruleus-cerebellum pathway block fear memory without impairing motor function. Thus, norepinephrine release in the cerebellum is modulated by experience and underlies aversive learning.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

