HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells
- PMID: 37563144
- PMCID: PMC10415338
- DOI: 10.1038/s41467-023-40227-8
HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells
Abstract
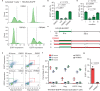
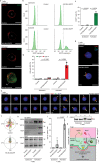
The mechanism of human immunodeficiency virus 1 (HIV-1) nuclear entry, required for productive infection, is not fully understood. Here, we report that in HeLa cells and activated CD4+ T cells infected with HIV-1 pseudotyped with VSV-G and native Env protein, respectively, Rab7+ late endosomes containing endocytosed HIV-1 promote the formation of nuclear envelope invaginations (NEIs) by a molecular mechanism involving the VOR complex, composed of the outer nuclear membrane protein VAP-A, hyperphosphorylated ORP3 and Rab7. Silencing VAP-A or ORP3 and drug-mediated impairment of Rab7 binding to ORP3-VAP-A inhibited the nuclear transfer of the HIV-1 components and productive infection. In HIV-1-resistant quiescent CD4+ T cells, ORP3 was not hyperphosphorylated and neither VOR complex nor NEIs were formed. This new cellular pathway and its molecular players are potential therapeutic targets, perhaps shared by other viruses that require nuclear entry to complete their life cycle.
© 2023. The Author(s).
Conflict of interest statement
The United Kingdom patent application GB2598624A (applicants: M.F.S., G.R., P.D., G.C., A.L., and Technische Universität Dresden; inventors: M.F.S., G.R., P.D., G.C., D.C., and A.L.), European patent application EP3864409A1 (applicants: M.F.S., G.R., A.L. and Technische Universität Dresden; inventors; M.F.S., G.R., D.C. and A.L.) and United States provisional patent number US20210353616A1 (applicants: M.F.S., G.R., A.L. and Technische Universität Dresden; inventors: M.F.S., G.R., D.C., and A.L.) are pending. The patent EP3864409A1/US20210353616A1 is entitled: Inhibition of a tripartite VOR protein complex in multicellular organisms. The patent GB2598624A is entitled: Use of triazole analogues for inhibition of a tripartite VOR protein complex in multicellular organism. These patents are related to the use of itraconazole and triazole analogues to inhibit the VOR protein complex, which prevents the nuclear transfer of materials transported by extracellular particles (e.g., extracellular vesicles and viruses). The authors declare no other competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

