Neuroblastoma RAS viral oncogene homolog (N-RAS) deficiency aggravates liver injury and fibrosis
- PMID: 37563155
- PMCID: PMC10415403
- DOI: 10.1038/s41419-023-06029-y
Neuroblastoma RAS viral oncogene homolog (N-RAS) deficiency aggravates liver injury and fibrosis
Abstract
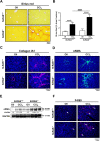
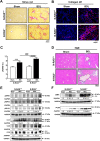
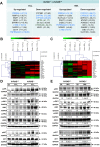
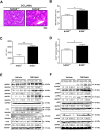
Progressive hepatic damage and fibrosis are major features of chronic liver diseases of different etiology, yet the underlying molecular mechanisms remain to be fully defined. N-RAS, a member of the RAS family of small guanine nucleotide-binding proteins also encompassing the highly homologous H-RAS and K-RAS isoforms, was previously reported to modulate cell death and renal fibrosis; however, its role in liver damage and fibrogenesis remains unknown. Here, we approached this question by using N-RAS deficient (N-RAS-/-) mice and two experimental models of liver injury and fibrosis, namely carbon tetrachloride (CCl4) intoxication and bile duct ligation (BDL). In wild-type (N-RAS+/+) mice both hepatotoxic procedures augmented N-RAS expression in the liver. Compared to N-RAS+/+ counterparts, N-RAS-/- mice subjected to either CCl4 or BDL showed exacerbated liver injury and fibrosis, which was associated with enhanced hepatic stellate cell (HSC) activation and leukocyte infiltration in the damaged liver. At the molecular level, after CCl4 or BDL, N-RAS-/- livers exhibited augmented expression of necroptotic death markers along with JNK1/2 hyperactivation. In line with this, N-RAS ablation in a human hepatocytic cell line resulted in enhanced activation of JNK and necroptosis mediators in response to cell death stimuli. Of note, loss of hepatic N-RAS expression was characteristic of chronic liver disease patients with fibrosis. Collectively, our study unveils a novel role for N-RAS as a negative controller of the progression of liver injury and fibrogenesis, by critically downregulating signaling pathways leading to hepatocyte necroptosis. Furthermore, it suggests that N-RAS may be of potential clinical value as prognostic biomarker of progressive fibrotic liver damage, or as a novel therapeutic target for the treatment of chronic liver disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

