Daratumumab monotherapy for refractory lupus nephritis
- PMID: 37563241
- PMCID: PMC10427415
- DOI: 10.1038/s41591-023-02479-1
Daratumumab monotherapy for refractory lupus nephritis
Abstract
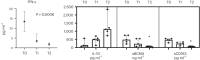
Treatment-refractory lupus nephritis (LN) has a high risk of a poor outcome and is often life-threatening. Here we report a case series of six patients (one male and five females) with a median age of 41.3 years (range, 20-61 years) with refractory LN who received renal biopsies and were subsequently treated with intravenous daratumumab, an anti-CD38 monoclonal antibody (weekly for 8 weeks, followed by eight biweekly infusions and up to eight monthly infusions). One patient did not show any improvement after 6 months of therapy, and daratumumab was discontinued. In five patients, the mean disease activity, as assessed by the Systemic Lupus Erythematosus Disease Activity 2000 index, decreased from 10.8 before treatment to 3.6 at 12 months after treatment. Mean proteinuria (5.6 g per 24 h to 0.8 g per 24 h) and mean serum creatinine (2.3 mg dl-1 to 1.5 mg dl-1) also decreased after 12 months. Improvement of clinical symptoms was accompanied by seroconversion of anti-double-stranded DNA antibodies; decreases in median interferon-gamma levels, B cell maturation antigen and soluble CD163 levels; and increases in C4 and interleukin-10 levels. These data suggest that daratumumab monotherapy warrants further exploration as a potential treatment for refractory LN.
© 2023. The Author(s).
Conflict of interest statement
The authors received no specific funding for this work. The authors declare no conflicts of interest and declare no support from any organizations for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

