A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection
- PMID: 37563299
- PMCID: PMC11073568
- DOI: 10.1038/s41587-023-01906-5
A germ-free humanized mouse model shows the contribution of resident microbiota to human-specific pathogen infection
Abstract
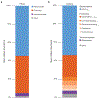
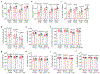
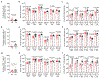
Germ-free (GF) mice, which are depleted of their resident microbiota, are the gold standard for exploring the role of the microbiome in health and disease; however, they are of limited value in the study of human-specific pathogens because they do not support their replication. Here, we develop GF mice systemically reconstituted with human immune cells and use them to evaluate the role of the resident microbiome in the acquisition, replication and pathogenesis of two human-specific pathogens, Epstein-Barr virus (EBV) and human immunodeficiency virus (HIV). Comparison with conventional (CV) humanized mice showed that resident microbiota enhance the establishment of EBV infection and EBV-induced tumorigenesis and increase mucosal HIV acquisition and replication. HIV RNA levels were higher in plasma and tissues of CV humanized mice compared with GF humanized mice. The frequency of CCR5+ CD4+ T cells throughout the intestine was also higher in CV humanized mice, indicating that resident microbiota govern levels of HIV target cells. Thus, resident microbiota promote the acquisition and pathogenesis of two clinically relevant human-specific pathogens.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures

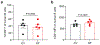



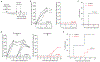

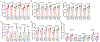
References
Methods-only References
MeSH terms
Grants and funding
- U19 AI082637/AI/NIAID NIH HHS/United States
- UM1 AI126619/AI/NIAID NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- P30DK034987/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DK131585/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- K24 AI143471/AI/NIAID NIH HHS/United States
- D43 TW009532/TW/FIC NIH HHS/United States
- AI123010/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30 DK056350/DK/NIDDK NIH HHS/United States
- 1UM1AI126619/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30 AI050410/AI/NIAID NIH HHS/United States
- R34 MH119963/MH/NIMH NIH HHS/United States
- U19AI082637/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P40 OD010995/OD/NIH HHS/United States
- P40OD010995/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- D43TW009532/U.S. Department of Health & Human Services | NIH | Fogarty International Center (FIC)
- R01 AI123010/AI/NIAID NIH HHS/United States
- UM1 AI164567/AI/NIAID NIH HHS/United States
- R01 DK131585/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

