Air pollution induces pyroptosis of human monocytes through activation of inflammasomes and Caspase-3-dependent pathways
- PMID: 37563611
- PMCID: PMC10416410
- DOI: 10.1186/s12950-023-00353-y
Air pollution induces pyroptosis of human monocytes through activation of inflammasomes and Caspase-3-dependent pathways
Abstract
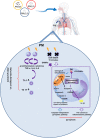
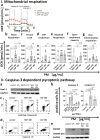
According to the World Health Organization (WHO), air pollution is one of the most serious threats for our planet. Despite a growing public awareness of the harmful effects of air pollution on human health, the specific influence of particulate matter (PM) on human immune cells remains poorly understood. In this study, we investigated the effect of PM on peripheral blood monocytes in vitro. Monocytes from healthy donors (HD) were exposed to two types of PM: NIST (SRM 1648a, standard urban particulate matter from the US National Institute for Standards and Technology) and LAP (SRM 1648a with the organic fraction removed). The exposure to PM-induced mitochondrial ROS production followed by the decrease of mitochondrial membrane potential and activation of apoptotic protease activating factor 1 (Apaf-1), Caspase-9, and Caspase-3, leading to the cleavage of Gasdermin E (GSDME), and initiation of pyroptosis. Further analysis showed a simultaneous PM-dependent activation of inflammasomes, including NLRP3 (nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3) and Caspase-1, followed by cleavage of Gasdermin D (GSDMD) and secretion of IL-1β. These observations suggest that PM-treated monocytes die by pyroptosis activated by two parallel signaling pathways, related to the inorganic and organic PM components. The release of IL-1β and expression of danger-associated molecular patterns (DAMPs) by pyroptotic cells further activated the remnant viable monocytes to produce inflammatory cytokines (TNF-α, IL-6, IL-8) and protected them from death induced by the second challenge with PM.In summary, our report shows that PM exposure significantly impacts monocyte function and induces their death by pyroptosis. Our observations indicate that the composition of PM plays a crucial role in this process-the inorganic fraction of PM is responsible for the induction of the Caspase-3-dependent pyroptotic pathway. At the same time, the canonical inflammasome path is activated by the organic components of PM, including LPS (Lipopolysaccharide/endotoxin). PM-induced pyroptosis of human monocytes. Particulate matter (PM) treatment affects monocytes viability already after 15 min of their exposure to NIST or LAP in vitro. The remnant viable monocytes in response to danger-associated molecular patterns (DAMPs) release pro-inflammatory cytokines and activate Th1 and Th17 cells. The mechanism of PM-induced cell death includes the increase of reactive oxygen species (ROS) production followed by collapse of mitochondrial membrane potential (ΔΨm), activation of Apaf-1, Caspase-9 and Caspase-3, leading to activation of Caspase-3-dependent pyroptotic pathway, where Caspase-3 cleaves Gasdermin E (GSDME) to produce a N-terminal fragment responsible for the switch from apoptosis to pyroptosis. At the same time, PM activates the canonical inflammasome pathway, where activated Caspase-1 cleaves the cytosolic Gasdermin D (GSDMD) to produce N-terminal domain allowing IL-1β secretion. As a result, PM-treated monocytes die by pyroptosis activated by two parallel pathways-Caspase-3-dependent pathway related to the inorganic fraction of PM and the canonical inflammasome pathway dependent on the organic components of PM.
Keywords: Cytokine production; NLRP3 inflammasome activation; Particulate matter; Peripheral blood monocytes; Pyroptosis.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome.Cell Rep. 2018 Nov 6;25(6):1525-1536.e7. doi: 10.1016/j.celrep.2018.09.071. Cell Rep. 2018. PMID: 30404007
-
NLRP3 inflammasome activation triggers gasdermin D-independent inflammation.Sci Immunol. 2021 Oct 22;6(64):eabj3859. doi: 10.1126/sciimmunol.abj3859. Epub 2021 Oct 22. Sci Immunol. 2021. PMID: 34678046 Free PMC article.
-
Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review.
-
Enterococcus Faecalis activates NLRP3 inflammasomes leading to increased interleukin-1 beta secretion and pyroptosis of THP-1 macrophages.Microb Pathog. 2021 May;154:104761. doi: 10.1016/j.micpath.2021.104761. Epub 2021 Jan 29. Microb Pathog. 2021. PMID: 33524566
-
Gasdermin D in pyroptosis.Acta Pharm Sin B. 2021 Sep;11(9):2768-2782. doi: 10.1016/j.apsb.2021.02.006. Epub 2021 Apr 28. Acta Pharm Sin B. 2021. PMID: 34589396 Free PMC article. Review.
Cited by
-
Dietary Zinc activates the Nrf2 signaling pathway to inhibit pyroptosis and attenuate the lung inflammatory response in COPD.Cytotechnology. 2025 Apr;77(2):62. doi: 10.1007/s10616-025-00725-7. Epub 2025 Feb 18. Cytotechnology. 2025. PMID: 39980839
-
Environmental Toxins and Oxidative Stress: The Link to Cardiovascular Diseases.Antioxidants (Basel). 2025 May 17;14(5):604. doi: 10.3390/antiox14050604. Antioxidants (Basel). 2025. PMID: 40427486 Free PMC article. Review.
-
Particulate Matter Induces Oxidative Stress and Ferroptosis in Human Lung Epithelial Cells.Toxics. 2024 Feb 19;12(2):161. doi: 10.3390/toxics12020161. Toxics. 2024. PMID: 38393256 Free PMC article.
-
Low serum double-stranded DNA levels are associated with higher survival rates in severe COPD patients.ERJ Open Res. 2024 Jul 15;10(4):00240-2024. doi: 10.1183/23120541.00240-2024. eCollection 2024 Jul. ERJ Open Res. 2024. PMID: 39010886 Free PMC article.
-
APAF1 Silencing Ameliorates Diabetic Retinopathy by Suppressing Inflammation, Oxidative Stress, and Caspase-3/GSDME-Dependent Pyroptosis.Diabetes Metab Syndr Obes. 2024 Apr 10;17:1635-1649. doi: 10.2147/DMSO.S449049. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38616988 Free PMC article.
References
-
- Ritz SA. Air pollution as a potential contributor to the “epidemic” of autoimmune disease. Med Hypotheses. 2010;74:110–117. - PubMed
-
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. - PubMed
-
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2:17–71. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

