Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis
- PMID: 37563639
- PMCID: PMC10413697
- DOI: 10.1186/s12943-023-01831-w
Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis
Abstract
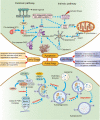
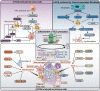
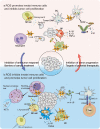
The reversible oxidation-reduction homeostasis mechanism functions as a specific signal transduction system, eliciting related physiological responses. Disruptions to redox homeostasis can have negative consequences, including the potential for cancer development and progression, which are closely linked to a series of redox processes, such as adjustment of reactive oxygen species (ROS) levels and species, changes in antioxidant capacity, and differential effects of ROS on downstream cell fate and immune capacity. The tumor microenvironment (TME) exhibits a complex interplay between immunity and regulatory cell death, especially autophagy and apoptosis, which is crucially regulated by ROS. The present study aims to investigate the mechanism by which multi-source ROS affects apoptosis, autophagy, and the anti-tumor immune response in the TME and the mutual crosstalk between these three processes. Given the intricate role of ROS in controlling cell fate and immunity, we will further examine the relationship between traditional cancer therapy and ROS. It is worth noting that we will discuss some potential ROS-related treatment options for further future studies.
Keywords: Apoptosis; Autophagy; Cancer therapy; Immunity; Redox and ROS.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Targeting ROS-Mediated Crosstalk Between Autophagy and Apoptosis in Cancer.Adv Exp Med Biol. 2020;1260:1-12. doi: 10.1007/978-3-030-42667-5_1. Adv Exp Med Biol. 2020. PMID: 32304028 Review.
-
Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response.Free Radic Biol Med. 2015 Dec;89:452-65. doi: 10.1016/j.freeradbiomed.2015.08.030. Epub 2015 Oct 9. Free Radic Biol Med. 2015. PMID: 26454086 Review.
-
Redox balance and autophagy regulation in cancer progression and their therapeutic perspective.Med Oncol. 2022 Nov 9;40(1):12. doi: 10.1007/s12032-022-01871-0. Med Oncol. 2022. PMID: 36352310 Review.
-
Reactive Oxygen Species: From Tumorigenesis to Therapeutic Strategies in Cancer.Cancer Med. 2025 May;14(10):e70947. doi: 10.1002/cam4.70947. Cancer Med. 2025. PMID: 40377005 Free PMC article. Review.
-
Autophagy: In the cROSshairs of cancer.Biochem Pharmacol. 2017 Feb 15;126:13-22. doi: 10.1016/j.bcp.2016.10.006. Epub 2016 Oct 24. Biochem Pharmacol. 2017. PMID: 27789215 Free PMC article. Review.
Cited by
-
A potential therapeutic strategy based on acute oxidative stress induction for wild-type NRF2/KEAP1 lung squamous cell carcinoma.Redox Biol. 2024 Sep;75:103305. doi: 10.1016/j.redox.2024.103305. Epub 2024 Aug 8. Redox Biol. 2024. PMID: 39137583 Free PMC article.
-
Identification of PANoptosis-related signature reveals immune infiltration characteristics and immunotherapy responses for renal cell carcinoma.BMC Cancer. 2024 Mar 4;24(1):292. doi: 10.1186/s12885-024-12067-2. BMC Cancer. 2024. PMID: 38439022 Free PMC article.
-
Comprehensive review of drug resistance in mammalian cancer stem cells: implications for cancer therapy.Cancer Cell Int. 2024 Dec 18;24(1):406. doi: 10.1186/s12935-024-03558-0. Cancer Cell Int. 2024. PMID: 39695669 Free PMC article. Review.
-
Metabolic Rewiring in the Face of Genomic Assault: Integrating DNA Damage Response and Cellular Metabolism.Biomolecules. 2025 Jan 23;15(2):168. doi: 10.3390/biom15020168. Biomolecules. 2025. PMID: 40001471 Free PMC article. Review.
-
Integration analysis using bioinformatics and experimental validation on cellular signalling for sex differences of hypertrophic cardiomyopathy.J Cell Mol Med. 2024 Nov;28(21):e70147. doi: 10.1111/jcmm.70147. J Cell Mol Med. 2024. PMID: 39535387 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

