The Basal Ganglia Downstream Control of Action - An Evolutionarily Conserved Strategy
- PMID: 37563813
- PMCID: PMC11097981
- DOI: 10.2174/1570159X21666230810141746
The Basal Ganglia Downstream Control of Action - An Evolutionarily Conserved Strategy
Abstract
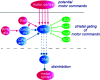
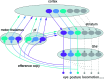
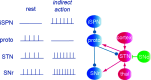
The motor areas of the cortex and the basal ganglia both contribute to determining which motor actions will be recruited at any moment in time, and their functions are intertwined. Here, we review the basal ganglia mechanisms underlying the selection of behavior of the downstream control of motor centers in the midbrain and brainstem and show that the basic organization of the forebrain motor system is evolutionarily conserved throughout vertebrate phylogeny. The output level of the basal ganglia (e.g. substantia nigra pars reticulata) has GABAergic neurons that are spontaneously active at rest and inhibit a number of specific motor centers, each of which can be relieved from inhibition if the inhibitory output neurons themselves become inhibited. The motor areas of the cortex act partially via the dorsolateral striatum (putamen), which has specific modules for the forelimb, hindlimb, trunk, etc. Each module operates in turn through the two types of striatal projection neurons that control the output modules of the basal ganglia and thereby the downstream motor centers. The mechanisms for lateral inhibition in the striatum are reviewed as well as other striatal mechanisms contributing to action selection. The motor cortex also exerts a direct excitatory action on specific motor centers. An overview is given of the basal ganglia control exerted on the different midbrain/brainstem motor centers, and the efference copy information fed back via the thalamus to the striatum and cortex, which is of importance for the planning of future movements.
Keywords: Evolution; basal ganglia; corticostriatal; dorsolateral striatum; motor centers; striatonigral.; substantia nigra pars reticulata.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures



References
-
- Ericsson J., Stephenson-Jones M., Pérez-Fernández J., Robertson B., Silberberg G., Grillner S. Dopamine differentially modulates the excitability of striatal neurons of the direct and indirect pathways in lamprey. J. Neurosci. 2013;33(18):8045–8054. doi: 10.1523/JNEUROSCI.5881-12.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- VR-M-K2013-62X-03026, VR-M-2015-02816, VR-M-2018-02453, VR-M-2019-01854, VR-M-2021-01995/Swedish Medical Research Council
- ITN-No-316639/EU/FP7 Moving Beyond
- FP7/2007-2013/European Union Seventh Framework Programme
- 604102, 720270, 785907, 945539/European Union's Horizon 2020 research and innovation program
- 860563/Marie Sklodowska-Curie (euSNN MSCA-ITN)
LinkOut - more resources
Full Text Sources
Miscellaneous

