Chemical insights into the synthetic chemistry of five-membered saturated heterocycles-a transition metal-catalyzed approach
- PMID: 37564110
- PMCID: PMC10411457
- DOI: 10.3389/fchem.2023.1185669
Chemical insights into the synthetic chemistry of five-membered saturated heterocycles-a transition metal-catalyzed approach
Abstract
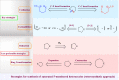
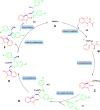
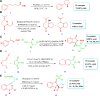
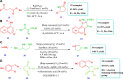
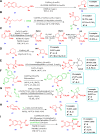
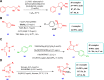
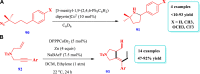
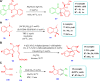
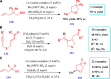
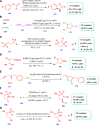
Drug design and delivery is primarily based on the hunt for new potent drug candidates and novel synthetic techniques. Recently, saturated heterocycles have gained enormous attention in medicinal chemistry as evidenced by the medicinal drugs listed in the FDA Orange Book. Therefore, the demand for novel saturated heterocyclic syntheses has increased tremendously. Transition metal (TM)-catalyzed reactions have remained the prime priority in heterocyclic syntheses for the last three decades. Nowadays, TM catalysis is well adorned by combining it with other techniques such as bio- and/or enzyme-catalyzed reactions, organocatalysis, or using two different metals in a single catalysis. This review highlights the recent developments of the transition metal-catalyzed synthesis of five-membered saturated heterocycles.
Keywords: catalysis; five-membered; green; heterocycles; saturated; synthesis; transition metals.
Copyright © 2023 Sunbal, Alamzeb, Omer, Abid, Ullah, Sohail and Ullah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




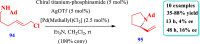








Similar articles
-
Transition metal-catalyzed C-H/C-C activation and coupling with 1,3-diyne.Org Biomol Chem. 2023 Apr 5;21(14):2842-2869. doi: 10.1039/d3ob00238a. Org Biomol Chem. 2023. PMID: 36917476 Review.
-
Recent advances in the synthesis of N-heteroarenes via catalytic dehydrogenation of N-heterocycles.Chem Commun (Camb). 2021 Dec 7;57(97):13042-13058. doi: 10.1039/d1cc04919d. Chem Commun (Camb). 2021. PMID: 34781335 Review.
-
Multi-component syntheses of heterocycles by transition-metal catalysis.Chem Soc Rev. 2007 Jul;36(7):1095-108. doi: 10.1039/b608235c. Epub 2007 Feb 19. Chem Soc Rev. 2007. PMID: 17576477 Review.
-
Alkynoates as Versatile and Powerful Chemical Tools for the Rapid Assembly of Diverse Heterocycles under Transition-Metal Catalysis: Recent Developments and Challenges.Top Curr Chem (Cham). 2021 Jan 5;379(1):3. doi: 10.1007/s41061-020-00316-4. Top Curr Chem (Cham). 2021. PMID: 33398642 Review.
-
Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles.Acc Chem Res. 2011 Nov 15;44(11):1156-71. doi: 10.1021/ar2000343. Epub 2011 Jul 29. Acc Chem Res. 2011. PMID: 21800828
Cited by
-
Fused-Linked and Spiro-Linked N-Containing Heterocycles.Int J Mol Sci. 2025 Aug 1;26(15):7435. doi: 10.3390/ijms26157435. Int J Mol Sci. 2025. PMID: 40806564 Free PMC article. Review.
-
An insight into recent developments of copper, silver and gold carbon dots: cancer diagnostics and treatment.Front Bioeng Biotechnol. 2023 Dec 14;11:1292641. doi: 10.3389/fbioe.2023.1292641. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38162182 Free PMC article. Review.
References
-
- Ahmad Khera R., Ahmad R., Ullah I., Abid O.-U.-R., Fatunsin O., Sher M., et al. (2010). Cyclization vs. Elimination reactions of 5-Aryl-5-hydroxy 1,3-diones: One-pot synthesis of 2-Aryl-2,3-dihydro-4H-pyran-4-ones. Helv. Chim. Acta 93 (9), 1705–1715. 10.1002/hlca.201000015 - DOI
-
- Ali A., Ullah I., Sher M., Villinger A., Langer P. (2009). Synthesis of sterically encumbered biaryls based on a ‘copper(I)-catalyzed arylation/[3+3] cyclocondensation’ strategy. Tetrahedron Lett. 50 (1), 118–120. 10.1016/j.tetlet.2008.10.093 - DOI
-
- Ali I., Siyo B., Hassan Z., Malik I., Ullah I., Ali A., et al. (2013). Synthesis of trifluoromethyl-substituted bi- and terphenyls by site-selective Suzuki–Miyaura reactions of various dihalogenated trifluoromethyl-benzene derivatives. J. Fluor. Chem. 145, 18–34. 10.1016/j.jfluchem.2012.11.005 - DOI
-
- Bagh B., Broere D. L. J., Sinha V., Kuijpers P. F., van Leest N. P., de Bruin B., et al. (2017). Catalytic synthesis of N-heterocycles via direct C(sp(3))-H amination using an air-stable iron(III) species with a redox-active ligand. J. Am. Chem. Soc. 139 (14), 5117–5124. 10.1021/jacs.7b00270 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

