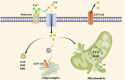
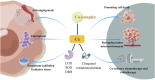
Targeting copper metabolism: a promising strategy for cancer treatment
- PMID: 37564178
- PMCID: PMC10411510
- DOI: 10.3389/fphar.2023.1203447
Targeting copper metabolism: a promising strategy for cancer treatment
Abstract
Copper is an essential micronutrient that plays a critical role in many physiological processes. However, excessive copper accumulation in cancer cells has been linked to tumor growth and metastasis. This review article explores the potential of targeting copper metabolism as a promising strategy for cancer treatment. Excessive copper accumulation in cancer cells has been associated with tumor growth and metastasis. By disrupting copper homeostasis in cancer cells and inducing cell death through copper-dependent mechanisms (cuproplasia and cuprotosis, respectively), therapies can be developed with improved efficacy and reduced side effects. The article discusses the role of copper in biological processes, such as angiogenesis, immune response, and redox homeostasis. Various approaches for targeting copper metabolism in cancer treatment are examined, including the use of copper-dependent enzymes, copper-based compounds, and cuprotosis-related genes or proteins. The review also explores strategies like copper chelation therapy and nanotechnology for targeted delivery of copper-targeting agents. By understanding the intricate network of cuprotosis and its interactions with the tumor microenvironment and immune system, new targets for therapy can be identified, leading to improved cancer treatment outcomes. Overall, this comprehensive review highlights the significant potential of targeting copper metabolism as a promising and effective approach in cancer treatment, while providing valuable insights into the current state of research in this field.
Keywords: cancer treatment; copper; cuprotosis; metabolism; tumor microenvironment.
Copyright © 2023 Kong and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Agarwal A., Kasinathan A., Ganesan R., Balasubramanian A., Bhaskaran J., Suresh S., et al. (2018). Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species–independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 51, 67–81. 10.1016/j.nutres.2017.12.011 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources