In vitro and in vivo effects of Pelargonium sidoides DC. root extract EPs® 7630 and selected constituents against SARS-CoV-2 B.1, Delta AY.4/AY.117 and Omicron BA.2
- PMID: 37564181
- PMCID: PMC10410074
- DOI: 10.3389/fphar.2023.1214351
In vitro and in vivo effects of Pelargonium sidoides DC. root extract EPs® 7630 and selected constituents against SARS-CoV-2 B.1, Delta AY.4/AY.117 and Omicron BA.2
Abstract
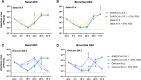
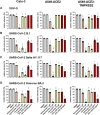
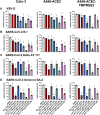
The occurrence of immune-evasive SARS-CoV-2 strains emphasizes the importance to search for broad-acting antiviral compounds. Our previous in vitro study showed that Pelargonium sidoides DC. root extract EPs® 7630 has combined antiviral and immunomodulatory properties in SARS-CoV-2-infected human lung cells. Here we assessed in vivo effects of EPs® 7630 in SARS-CoV-2-infected hamsters, and investigated properties of EPs® 7630 and its functionally relevant constituents in context of phenotypically distinct SARS-CoV-2 variants. We show that EPs® 7630 reduced viral load early in the course of infection and displayed significant immunomodulatory properties positively modulating disease progression in hamsters. In addition, we find that EPs® 7630 differentially inhibits SARS-CoV-2 variants in nasal and bronchial human airway epithelial cells. Antiviral effects were more pronounced against Omicron BA.2 compared to B.1 and Delta, the latter two preferring TMPRSS2-mediated fusion with the plasma membrane for cell entry instead of receptor-mediated low pH-dependent endocytosis. By using SARS-CoV-2 Spike VSV-based pseudo particles (VSVpp), we confirm higher EPs® 7630 activity against Omicron Spike-VSVpp, which seems independent of the serine protease TMPRSS2, suggesting that EPs® 7630 targets endosomal entry. We identify at least two molecular constituents of EPs® 7630, i.e., (-)-epigallocatechin and taxifolin with antiviral effects on SARS-CoV-2 replication and cell entry. In summary, our study shows that EPs® 7630 ameliorates disease outcome in SARS-CoV-2-infected hamsters and has enhanced activity against Omicron, apparently by limiting late endosomal SARS-CoV-2 entry.
Keywords: COVID-19; EPs 7630; Pelargonium sidoides; SARS-CoV-2; coronavirus; cytokine storm; drug repurposing; immune modulation.
Copyright © 2023 Emanuel, Papies, Galander, Adler, Heinemann, Eschke, Merz, Pischon, Rose, Krumbholz, Kulić, Lehner, Trimpert and Müller.
Conflict of interest statement
Authors ŽK and ML were employees by Dr. Willmar Schwabe GmbH and Co. KG. The funder had the following involvement with the study: ML contributed to the design of the study, analyzed data, provided material, wrote and edited the main text. ŽK provided material, analyzed data, wrote and edited the main text. SM and HP were employed by IDEXX Laboratories. AK was employed by Labor Dr. Krause und Kollegen MVZ GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Unlocking the therapeutic potential of Pelargonium sidoides natural extract: A scoping review.Heliyon. 2024 Nov 20;10(23):e40554. doi: 10.1016/j.heliyon.2024.e40554. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39654721 Free PMC article.
-
Antiviral and Immunomodulatory Effects of Pelargonium sidoides DC. Root Extract EPs® 7630 in SARS-CoV-2-Infected Human Lung Cells.Front Pharmacol. 2021 Oct 25;12:757666. doi: 10.3389/fphar.2021.757666. eCollection 2021. Front Pharmacol. 2021. PMID: 34759825 Free PMC article.
-
Corrigendum: Antiviral and Immunomodulatory Effects of Pelargonium sidoides DC. Root Extract EPs® 7630 in SARS-CoV-2-Infected Human Lung Cells.Front Pharmacol. 2021 Dec 17;12:814452. doi: 10.3389/fphar.2021.814452. eCollection 2021. Front Pharmacol. 2021. PMID: 34975509 Free PMC article.
-
SARS-CoV-2 Omicron entry is type II transmembrane serine protease-mediated in human airway and intestinal organoid models.J Virol. 2023 Aug 31;97(8):e0085123. doi: 10.1128/jvi.00851-23. Epub 2023 Aug 9. J Virol. 2023. PMID: 37555660 Free PMC article.
-
Therapeutic potential of green tea catechin, (-)-epigallocatechin-3-O-gallate (EGCG) in SARS-CoV-2 infection: Major interactions with host/virus proteases.Phytomed Plus. 2023 Feb;3(1):100402. doi: 10.1016/j.phyplu.2022.100402. Epub 2022 Dec 30. Phytomed Plus. 2023. PMID: 36597465 Free PMC article. Review.
Cited by
-
Unlocking the therapeutic potential of Pelargonium sidoides natural extract: A scoping review.Heliyon. 2024 Nov 20;10(23):e40554. doi: 10.1016/j.heliyon.2024.e40554. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39654721 Free PMC article.
-
Phytotherapy for acute respiratory tract infections in children: a systematically conducted, comprehensive review.Front Pediatr. 2025 May 1;13:1423250. doi: 10.3389/fped.2025.1423250. eCollection 2025. Front Pediatr. 2025. PMID: 40376625 Free PMC article.
-
Stevens' Cure (Umckaloabo)-the vindication of a patent medicine.Front Pharmacol. 2024 Jan 3;14:1294997. doi: 10.3389/fphar.2023.1294997. eCollection 2023. Front Pharmacol. 2024. PMID: 38235116 Free PMC article.
-
Long-term benefits of EPs® 7630 in patients with acute sinusitis: a real-world cohort study.Front Pharmacol. 2024 Mar 18;15:1358879. doi: 10.3389/fphar.2024.1358879. eCollection 2024. Front Pharmacol. 2024. PMID: 38562459 Free PMC article.
-
Identifying in-market application of Pelargonium root extract EPs 7630 for the treatment of COVID-19: analysis of pharmacovigilance data.Front Pharmacol. 2024 Feb 23;15:1335309. doi: 10.3389/fphar.2024.1335309. eCollection 2024. Front Pharmacol. 2024. PMID: 38464728 Free PMC article.
References
-
- Bertzbach L. D., Vladimirova D., Dietert K., Abdelgawad A., Gruber A. D., Osterrieder N., et al. (2021). SARS-CoV-2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID-19 pneumonia in a well-established small animal model. Transbound. Emerg. Dis. 68, 1075–1079. 10.1111/tbed.13837 - DOI - PMC - PubMed
-
- Biesold S. E., Ritz D., Gloza-Rausch F., Wollny R., Drexler J. F., Corman V. M., et al. (2011). Type I interferon reaction to viral infection in interferon-competent, immortalized cell lines from the African fruit bat Eidolon helvum . PloS one 6, e28131. 10.1371/journal.pone.0028131 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

