B7-H3 in Brain Malignancies: Immunology and Immunotherapy
- PMID: 37564196
- PMCID: PMC10411461
- DOI: 10.7150/ijbs.85813
B7-H3 in Brain Malignancies: Immunology and Immunotherapy
Abstract
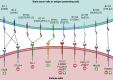
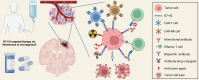
The immune checkpoint B7-H3 (CD276), a member of the B7 family with immunoregulatory properties, has been identified recently as a novel target for immunotherapy for refractory blood cancers and solid malignant tumors. While research on B7-H3 in brain malignancies is limited, there is growing interest in exploring its therapeutic potential in this context. B7-H3 plays a crucial role in regulating the functions of immune cells, cancer-associated fibroblasts, and endothelial cells within the tumor microenvironment, contributing to the creation of a pro-tumorigenic milieu. This microenvironment promotes uncontrolled cancer cell proliferation, enhanced metabolism, increased cancer stemness, and resistance to standard treatments. Blocking B7-H3 and terminating its immunosuppressive function is expected to improve anti-tumor immune responses and, in turn, ameliorate the progression of tumors. Results from preclinical or observative studies and early-phase trials targeting B7-H3 have revealed promising anti-tumor efficacy and acceptable toxicity in glioblastoma (GBM), diffuse intrinsic pontine glioma (DIPG), medulloblastoma, neuroblastoma, craniopharyngioma, atypical teratoid/rhabdoid tumor, and brain metastases. Ongoing clinical trials are now investigating the use of CAR-T cell therapy and antibody-drug conjugate therapy, either alone or in combination with standard treatments or other therapeutic approaches, targeting B7-H3 in refractory or recurrent GBMs, DIPGs, neuroblastomas, medulloblastomas, ependymomas, and metastatic brain tumors. These trials hold promise for providing effective treatment options for these challenging intracranial malignancies in both adult and pediatric populations.
Keywords: B7-H3; antibody-drug conjugate therapy; brain tumor; cancer immunotherapy; chimeric antigen receptor T cell therapy; tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Chapoval AI, Ni J, Lau JS. et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. - PubMed
-
- Kanchan RK, Doss D, Khan P. et al. To kill a cancer: Targeting the immune inhibitory checkpoint molecule, B7-H3. Biochim Biophys Acta Rev Cancer. 2022;1877:188783.. doi: 10.1016/j.bbcan.2022.188783. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

