Pulmonary surfactant and COVID-19: A new synthesis
- PMID: 37564950
- PMCID: PMC10411325
- DOI: 10.1017/qrd.2022.1
Pulmonary surfactant and COVID-19: A new synthesis
Abstract
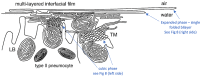
Chapter 1: COVID-19 pathogenesis poses paradoxes difficult to explain with traditional physiology. For instance, since type II pneumocytes are considered the primary cellular target of SARS-CoV-2; as these produce pulmonary surfactant (PS), the possibility that insufficient PS plays a role in COVID-19 pathogenesis has been raised. However, the opposite of predicted high alveolar surface tension is found in many early COVID-19 patients: paradoxically normal lung volumes and high compliance occur, with profound hypoxemia. That 'COVID anomaly' was quickly rationalised by invoking traditional vascular mechanisms-mainly because of surprisingly preserved alveolar surface in early hypoxemic cases. However, that quick rejection of alveolar damage only occurred because the actual mechanism of gas exchange has long been presumed to be non-problematic, due to diffusion through the alveolar surface. On the contrary, we provide physical chemical evidence that gas exchange occurs by an process of expansion and contraction of the three-dimensional structures of PS and its associated proteins. This view explains anomalous observations from the level of cryo-TEM to whole individuals. It encompasses results from premature infants to the deepest diving seals. Once understood, the COVID anomaly dissolves and is straightforwardly explained as covert viral damage to the 3D structure of PS, with direct treatment implications. As a natural experiment, the SARS-CoV-2 virus itself has helped us to simplify and clarify not only the nature of dyspnea and its relationship to pulmonary compliance, but also the fine detail of the PS including such features as water channels which had heretofore been entirely unexpected.
Chapter 2: For a long time, physical, colloid and surface chemistry have not intersected with physiology and cell biology as much as we might have hoped. The reasons are starting to become clear. The discipline of physical chemistry suffered from serious unrecognised omissions that rendered it ineffective. These foundational defects included omission of specific ion molecular forces and hydration effects. The discipline lacked a predictive theory of self-assembly of lipids and proteins. Worse, theory omitted any role for dissolved gases, O2, N2, CO2, and their existence as stable nanobubbles above physiological salt concentration. Recent developments have gone some way to explaining the foam-like lung surfactant structures and function. It delivers O2/N2 as nanobubbles, and efflux of CO2, and H2O nanobubbles at the alveolar surface. Knowledge of pulmonary surfactant structure allows an explanation of the mechanism of corona virus entry, and differences in infectivity of different variants. CO2 nanobubbles, resulting from metabolism passing through the molecular frit provided by the glycocalyx of venous tissue, forms the previously unexplained foam which is the endothelial surface layer. CO2 nanobubbles turn out to be lethal to viruses, providing a plausible explanation for the origin of 'Long COVID'. Circulating nanobubbles, stable above physiological 0.17 M salt drive various enzyme-like activities and chemical reactions. Awareness of the microstructure of Pulmonary Surfactant and that nanobubbles of (O2/N2) and CO2 are integral to respiratory and circulatory physiology provides new insights to the COVID-19 and other pathogen activity.
Keywords: COVID; Gas Exchange; Lung Surfactant; Pulmonary Surfactant; nano-bubbles; physical chemistry.
© The Author(s) 2022.
Figures











References
Chapter 1
-
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ and Jonigk D (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. The New England Journal of Medicine 383, 120–128. 10.1056/NEJMoa2015432. - DOI - PMC - PubMed
-
- Ahmetaj-Shala B, Peacock P, Baillon L, Swann OC, Gashaw H, Barclay WS and Mitchell JA (2021) Resistance of endothelial cells to SARS-CoV-2 infection in vitro. bioRxiv. 10.1101/2020.11.08.372581. - DOI
Chapter 2
The Background
-
- Lo Nostro P and Ninham BW (2020) After DLVO: Hans Lyklema and the keepers of the faith. Advances in Colloid and Interface Science 276, 102082. - PubMed
-
- Ninham BW (2006) The present state of molecular forces. Progress in Colloid and Polymer Science 133, 65–73. 10.1007/2882_051. - DOI
-
- Ninham BW (2017) The biological/physical sciences divide and the age of unreason. Substantia 1(1), 7–24. 10.13128/Substantia-6. - DOI
-
- Ninham BW (2019) B. V. Derjaguin and J. Theo. G. Overbeek. Their times, and ours. Substantia 3(2), 65–72. 10.13128/Substantia-637. - DOI
-
- Ninham BW and Lo Nostro P (2010) Molecular Forces and Self-Assembly: In Colloid, Nano Sciences and Biology. Cambridge: Cambridge University Press.
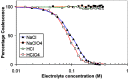
Bubble–bubble fusion in electrolytes
-
- Craig V and Henry C (2010) Inhibition of bubble coalescence by salts and sugars. In XXV International Mineral Processing Congress: IMPC 2010 Proceedings, Brisbane, Australia, ed. Conference Program Committee, CSIRO Publishing pp. 1814–1826.
-
- Henry CL and Craig VSG (2009) Inhibition of bubble coalescence by osmolytes: Sucrose, other sugars, and urea. Langmuir 25, 11406–11412. - PubMed
-
- Kékicheff P and Ninham BW (1990) The double-layer interaction of asymmetric electrolytes. Europhysics Letters 12(5), 471–477. 10.1209/0295-5075/12/5/016. - DOI
-
- Nylander T, Kékicheff P and Ninham BW (1994) The effect of solution behavior of insulin on interactions between adsorbed layers of insulin. Journal of Colloid and Interface Science 164(1), 136–150. 10.1006/jcis.1994.1152. - DOI
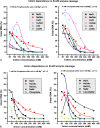
Classical electrolyte theory and colloid science
-
- Boström M, Craig VJS, Albion R, Williams DRM and Ninham BW (2003) Hofmeister effects in pH measurements: Role of added salt and co-ions. The Journal of Physical Chemistry. B 107(13), 2875–2878. 10.1021/jp026804d. - DOI
-
- Ninham BW, Kurihara K and Vinogradova OI (1997) Hydrophobicity, specific ion adsorption and reactivity. Colloids Surfaces A: Physicochemical and Engineering Aspects 123–124, 7–12. 10.1016/S0927-7757(96)03794-6. - DOI
Dissolved atmospheric gas
-
- Ninham BW and Pashley RM (2020) ‘Outline’, ‘Postscript’, & ‘cavitation’, in ‘about water: Novel technologies for the new millennium’. special issue of Substantia 4(2 Suppl.), 5–8. 10.36253/Substantia-1155. - DOI
-
- Taseidifar M, Antony J and Pashley RM (2020) Prevention of cavitation in propellers. Substantia 4(2 Suppl.), 109–117. 10.36253/Substantia-821. - DOI
Nanobubbles
-
- Alheshibri M and Craig VSJ (2018) Differentiating between nanoparticles and nanobubbles by evaluation of the compressibility and density of nanoparticles. The Journal of Physical Chemistry C 122, 21998–22007. 10.1021/acs.jpcc.8b07174. - DOI
-
- Bunkin NF, Kiseleva OA, Lobeyev AV, Movchan TG, Ninham BW and Vinogradova OI (1997) Effect of salts and dissolved gas on optical cavitation near hydrophobic and hydrophilic surfaces. Langmuir 13(11), 3024–3028. 10.1021/la960265k. - DOI
Carbon dioxide bubbles destroy viruses and bacteria: CO2 a saint not sinner
-
- Arkill KP, Knupp C, Michel CC, Neal CR, Qvortrup K, Rostgaard J and Squire JM (2011) Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: Evidence for a common filtering mechanism? Biophysical Journal 101(5), 1046–1056. 10.1016/j.bpj.2011.07.036. - DOI - PMC - PubMed
Chemical reactions, enzyme action, nanobubbles
-
- Allen M, Evans DF, Mitchell DJ and Ninham BW (1987) Interfacial tension of ionic microemulsions. Journal of Physical Chemistry 91(9), 2320–2324. 10.1021/j100293a022. - DOI
-
- Evans DF, Mitchell DJ and Ninham BW (1986) Oil, water, and surfactant: Properties and conjectured structure of simple microemulsions. The Journal of Physical Chemistry 90(13), 2817–2825. 10.1021/j100404a009. - DOI
-
- Feng B, Sosa RP, Mårtensson AKF, Jiang K, Tong A, Dorfman KD, Takahashi M, Lincoln P, Bustamante CJ, Westerlund F and Nordén B (2019) Hydrophobic catalysis and a potential biological role of DNA unstacking induced by environment effects. Proceedings of the National Academy of Sciences of the United States of America 116, 17169–17174. - PMC - PubMed
-
- Knackstedt MA and Ninham BW (1995) Ternary microemulsions as model disordered media. AICHE Journal 41(5), 1295–1305. 10.1002/aic.690410524. - DOI
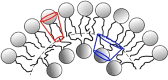
Lung surfactant structure: Self-assembly of lipids
-
- Bangham AD (1995) Surface tension in the lungs. Biophysical Journal 68, 1630–1633. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1282060/pdf/biophysj00063-0.... - PMC - PubMed
-
- Follows D, Tiberg F, Thomas RK and Larsson M (2007) Multilayers at the surface of solutions of exogenous lung surfactant: Direct observation by neutron reflection. Biochimica et Biophysica Acta 1768, 228–235. - PubMed
The real lung surfactant – Structure and machinery
-
- Angelova A, Angelov B, Drechsler M, Bizien T, Gorshkova YE and Deng Y (2021) Plasmalogen-based liquid crystalline multiphase structures involving Docosapentaenoyl derivatives inspired by biological cubic membranes. Frontiers in Cell and Developmental Biology 9, 617984. 10.3389/fcell.2021.617984. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
