Assessment of antibodies in the upper and lower human respiratory tract at steady state and after respiratory viral infection
- PMID: 37564999
- PMCID: PMC10410120
- DOI: 10.1002/cti2.1460
Assessment of antibodies in the upper and lower human respiratory tract at steady state and after respiratory viral infection
Abstract
Objectives: There is an increasing appreciation for the need to study mucosal antibody responses in humans. Our aim was to determine the utility of different types of samples from the human respiratory tract, specifically nasopharyngeal (NP) swabs obtained for diagnostic purposes and bronchoalveolar lavage (BAL) obtained in outpatient and inpatient settings.
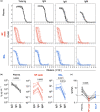
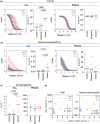
Methods: We analysed antibody levels in plasma and NP swabs from 67 individuals with acute influenza as well as plasma and BAL from individuals undergoing bronchoscopy, including five control subjects as well as seven moderately and seven severely ill subjects with a respiratory viral infection. Levels of α2-macroglobulin were determined in BAL and plasma to assess plasma exudation.
Results: IgG and IgA were readily detectable in BAL and NP swabs, albeit at different ratios, while IgM levels were low. The total amount of antibody recovered from NP swabs varied greatly between study participants. Accordingly, the levels of influenza HA-specific antibodies varied, and individuals with lower amounts of total Ig in NP swabs had undetectable levels of HA-specific Ig. Similarly, the total amount of antibody recovered from BAL varied between study participants. However, severely ill patients showed evidence of increased plasma exudation, which may confound analysis of their BAL samples for mucosal antibodies.
Conclusion: Nasopharyngeal swabs collected for diagnostic purposes may have utility in assessing antibodies from the human nasal mucosa, but variability in sampling should be accounted for. BAL samples can be utilised to study antibodies from the lower respiratory tract, but the possibility of plasma exudation should be excluded.
Keywords: BAL; antibodies; mucosal immunity; nasopharyngeal swab.
© 2023 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The Ellebedy laboratory has received funding from Moderna, Emergent BioSolutions and AbbVie, which are unrelated to the data presented in the current study. JST has received consulting fees from Curevac. AHE has received consulting and speaking fees from InBios International, Inc, Fimbrion Therapeutics, RGAX, Mubadala Investment Company, Moderna, Pfizer, GSK, Danaher, Third Rock Ventures, Goldman Sachs and Morgan Stanley and is the founder of ImmuneBio Consulting. JST and AHE are recipients of a licensing agreement with Abbvie that is unrelated to the data presented in the current study. Other authors declare no conflicts of interest.
Figures

References
-
- Khoury DS, Cromer D, Reynaldi A et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med 2021; 27: 1205–1211. - PubMed
-
- Planas D, Bruel T, Grzelak L et al. Sensitivity of infectious SARS‐CoV‐2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med 2021; 27: 917–924. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
