Establishment of a mathematical prediction model for voriconazole stable maintenance dose: a prospective study
- PMID: 37565064
- PMCID: PMC10410275
- DOI: 10.3389/fcimb.2023.1157944
Establishment of a mathematical prediction model for voriconazole stable maintenance dose: a prospective study
Abstract
Background: In patients with invasive fungal infection (IFI), the steady-state serum trough concentration (C min) of voriconazole (VCZ) is highly variable and can lead to treatment failure (C min < 0.5 mg/L) and toxicity (C min ≥ 5.0 mg/L). However, It remains challenging to determine the ideal maintenance dose to achieve the desired C min level quickly.
Aims: This randomized, prospective observational single-center study aimed to identify factors affecting VCZ-C min and maintenance dose and create an algorithmic model to predict the necessary maintenance dose. MeThe study enrolled 306 adult IFI patients, split into two groups: non-gene-directed (A) (where CYP2C19 phenotype is not involved in determining VCZ dose) and gene-directed (B) (where CYP2C19 phenotype is involved in determining VCZ dose).
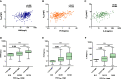
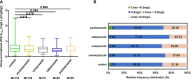
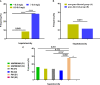
Results: Results indicated that CYP2C19 genetic polymorphisms might significantly impact VCZ loading and maintenance dose selection. CYP2C19 phenotype, C-reaction protein (CRP), and average daily dose/body weight were significant influencers on VCZ-C min, while CYP2C19 phenotype, CRP, and body weight significantly impacted VCZ maintenance dose. A feasible predictive formula for VCZ stable maintenance dose was derived from the regression equation as a maintenance dose (mg) =282.774-0.735×age (year)+2.946×body weight(Kg)-19.402×CYP2C19 phenotype (UM/RM/NM:0, IM:1, PM:2)-0.316×CRP (mg/L) (p < 0.001).
Discussion: DiThis formula may serve as a valuable supplement to the Clinical Pharmacogenetics Implementation Consortium (CPIC®) guideline for CYP2C19 and VCZ therapy, especially for IFI patients with highly variable inflammatory cytokines during VCZ therapy.
Keywords: CYP2C19; prediction model; proinflammatory cytokines; security; voriconazole.
Copyright © 2023 Zhou, Li, Li, Guo, Gao, Zhang, Qin, Sang, Xing, Cheng and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Blanco Dorado S., Maroñas Amigo O., Latorre-Pellicer A., Rodriguez Jato M. T., Lopez-Vizcaino A., Gomez Marquez A., et al. (2020). A multicentre prospective study evaluating the impact of proton-pump inhibitors omeprazole and pantoprazole on voriconazole plasma concentrations. Br. J. Clin. Pharmacol. 86 (8), 1661–1666. doi: 10.1111/bcp.14267 - DOI - PMC - PubMed
-
- Bolcato L., Khouri C., Veringa A., Alffenaar J. W. C., Yamada T., Naito T., et al. (2021). Combined impact of inflammation and pharmacogenomic variants on voriconazole trough concentrations: A meta-analysis of individual data. J. Clin. Med. 10 (10), 2089. doi: 10.3390/jcm10102089 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

