CCND2 Modified mRNA Activates Cell Cycle of Cardiomyocytes in Hearts With Myocardial Infarction in Mice and Pigs
- PMID: 37565345
- PMCID: PMC10529295
- DOI: 10.1161/CIRCRESAHA.123.322929
CCND2 Modified mRNA Activates Cell Cycle of Cardiomyocytes in Hearts With Myocardial Infarction in Mice and Pigs
Abstract
Background: Experiments in mammalian models of cardiac injury suggest that the cardiomyocyte-specific overexpression of CCND2 (cyclin D2, in humans) improves recovery from myocardial infarction (MI). The primary objective of this investigation was to demonstrate that our specific modified mRNA translation system (SMRTs) can induce CCND2 expression in cardiomyocytes and replicate the benefits observed in other studies of cardiomyocyte-specific CCND2 overexpression for myocardial repair.
Methods: The CCND2-cardiomyocyte-specific modified mRNA translation system (cardiomyocyte SMRTs) consists of 2 modRNA constructs: one codes for CCND2 and contains a binding site for L7Ae, and the other codes for L7Ae and contains recognition elements for the cardiomyocyte-specific microRNAs miR-1 and miR-208. Thus, L7Ae suppresses CCND2 translation in noncardiomyocytes but is itself suppressed by endogenous miR-1 and -208 in cardiomyocytes, thereby facilitating cardiomyocyte-specific CCND2 expression. Experiments were conducted in both mouse and pig models of MI, and control assessments were performed in animals treated with an SMRTs coding for the cardiomyocyte-specific expression of luciferase or green fluorescent protein (GFP), in animals treated with L7Ae modRNA alone or with the delivery vehicle, and in Sham-operated animals.
Results: CCND2 was abundantly expressed in cultured, postmitotic cardiomyocytes 2 days after transfection with the CCND2-cardiomyocyte SMRTs, and the increase was accompanied by the upregulation of markers for cell-cycle activation and proliferation (eg, Ki67 and Aurora B kinase). When the GFP-cardiomyocyte SMRTs were intramyocardially injected into infarcted mouse hearts, the GFP signal was observed in cardiomyocytes but no other cell type. In both MI models, cardiomyocyte proliferation (on day 7 and day 3 after treatment administration in mice and pigs, respectively) was significantly greater, left-ventricular ejection fractions (days 7 and 28 in mice, days 10 and 28 in pigs) were significantly higher, and infarcts (day 28 in both species) were significantly smaller in animals treated with the CCND2-cardiomyocyte SMRTs than in any other group that underwent MI induction.
Conclusions: Intramyocardial injections of the CCND2-cardiomyocyte SMRTs promoted cardiomyocyte proliferation, reduced infarct size, and improved cardiac performance in small and large mammalian hearts with MI.
Keywords: cardiomyocyte; cell cycle; heart failure; myocardial infarction.
Conflict of interest statement
Figures
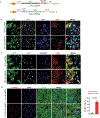


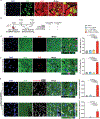

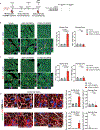
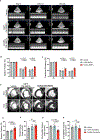





Comment in
-
Will Induction of Transient Myocyte Proliferation Be a Regenerative Therapy?Circ Res. 2023 Sep;133(6):505-507. doi: 10.1161/CIRCRESAHA.123.323399. Epub 2023 Aug 31. Circ Res. 2023. PMID: 37651544 No abstract available.
References
-
- Nakada Y, Zhou Y, Gong W, Zhang EY, Skie E, Nguyen T, Wei Y, Zhao M, Chen W, Sun J, et al. Single Nucleus Transcriptomics: Apical Resection in Newborn Pigs Extends the Time Window of Cardiomyocyte Proliferation and Myocardial Regeneration. Circulation. 2022;145:1744–1747. doi: 10.1161/circulationaha.121.056995 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

