Protein O-mannosylation: one sugar, several pathways, many functions
- PMID: 37565810
- PMCID: PMC10859634
- DOI: 10.1093/glycob/cwad067
Protein O-mannosylation: one sugar, several pathways, many functions
Abstract
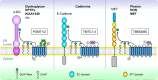
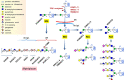
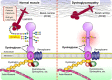
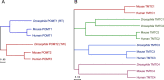
Recent research has unveiled numerous important functions of protein glycosylation in development, homeostasis, and diseases. A type of glycosylation taking the center stage is protein O-mannosylation, a posttranslational modification conserved in a wide range of organisms, from yeast to humans. In animals, protein O-mannosylation plays a crucial role in the nervous system, whereas protein O-mannosylation defects cause severe neurological abnormalities and congenital muscular dystrophies. However, the molecular and cellular mechanisms underlying protein O-mannosylation functions and biosynthesis remain not well understood. This review outlines recent studies on protein O-mannosylation while focusing on the functions in the nervous system, summarizes the current knowledge about protein O-mannosylation biosynthesis, and discusses the pathologies associated with protein O-mannosylation defects. The evolutionary perspective revealed by studies in the Drosophila model system are also highlighted. Finally, the review touches upon important knowledge gaps in the field and discusses critical questions for future research on the molecular and cellular mechanisms associated with protein O-mannosylation functions.
Keywords: Drosophila model system; matriglycan; protein O-mannosylation; protein O-mannosyltransferases; receptor protein tyrosine phosphatase.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Ackroyd MR, Skordis L, Kaluarachchi M, Godwin J, Prior S, Fidanboylu M, Piercy RJ, Muntoni F, Brown SC. Reduced expression of fukutin related protein in mice results in a model for fukutin related protein associated muscular dystrophies. Brain. 2009:132:439–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

