Genetic Downregulation of the Metabotropic Glutamate Receptor Type 5 Dampens the Reactive and Neurotoxic Phenotype of Adult ALS Astrocytes
- PMID: 37566031
- PMCID: PMC10416852
- DOI: 10.3390/cells12151952
Genetic Downregulation of the Metabotropic Glutamate Receptor Type 5 Dampens the Reactive and Neurotoxic Phenotype of Adult ALS Astrocytes
Abstract
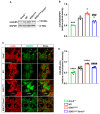
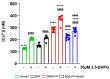
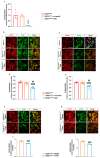
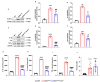
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive degeneration of motor neurons (MNs). Astrocytes display a toxic phenotype in ALS, which results in MN damage. Glutamate (Glu)-mediated excitotoxicity and group I metabotropic glutamate receptors (mGluRs) play a pathological role in the disease progression. We previously demonstrated that in vivo genetic ablation or pharmacological modulation of mGluR5 reduced astrocyte activation and MN death, prolonged survival and ameliorated the clinical progression in the SOD1G93A mouse model of ALS. This study aimed to investigate in vitro the effects of mGluR5 downregulation on the reactive spinal cord astrocytes cultured from adult late symptomatic SOD1G93A mice. We observed that mGluR5 downregulation in SOD1G93A astrocytes diminished the cytosolic Ca2+ overload under resting conditions and after mGluR5 simulation and reduced the expression of the reactive glial markers GFAP, S100β and vimentin. In vitro exposure to an anti-mGluR5 antisense oligonucleotide or to the negative allosteric modulator CTEP also ameliorated the altered reactive astrocyte phenotype. Downregulating mGluR5 in SOD1G93A mice reduced the synthesis and release of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α and ameliorated the cellular bioenergetic profile by improving the diminished oxygen consumption and ATP synthesis and by lowering the excessive lactate dehydrogenase activity. Most relevantly, mGluR5 downregulation hampered the neurotoxicity of SOD1G93A astrocytes co-cultured with spinal cord MNs. We conclude that selective reduction in mGluR5 expression in SOD1G93A astrocytes positively modulates the astrocyte reactive phenotype and neurotoxicity towards MNs, further supporting mGluR5 as a promising therapeutic target in ALS.
Keywords: Grm5 genetic ablation; SOD1G93A mice; adult mouse spinal cord astrocytes; amyotrophic lateral sclerosis; mGlu5 receptor.
Conflict of interest statement
The authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. P.J.-n. and F.R. are paid employees of Ionis Pharmaceuticals.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

