Preparation and Characterization of 3D-Printed Dose-Flexible Printlets of Tenofovir Disoproxil Fumarate
- PMID: 37566167
- PMCID: PMC12047738
- DOI: 10.1208/s12249-023-02623-7
Preparation and Characterization of 3D-Printed Dose-Flexible Printlets of Tenofovir Disoproxil Fumarate
Abstract
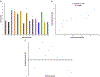
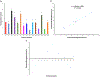
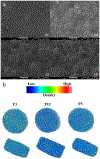
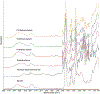
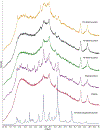
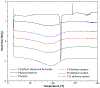
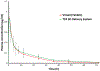
The aim of this work was to design pediatric-friendly, dose-flexible orally disintegrating drug delivery systems (printlets) of the antiviral drug tenofovir disoproxil fumarate (TDF) by selective laser sintering (SLS) for potential use in hospitals along with other antiviral drugs. In order to obtain a consistent quality of printlets with desired properties, it is important to understand certain critical quality attributes for their main and interactions effect. The printlets were optimized by Box-Behnken's design of the experiment by varying process variables while keeping the composition constant. The composition contained 16.3% TDF, 72.7% polyvinyl pyrrolidone K16-18, 8% magnesium aluminum silicate, 3% Candurin® NXT Ruby Red, and 0.3% colloidal silicon dioxide. The process variables studied were surface (X1), chamber temperatures (X2), and laser scanning speed (X3). The range of variable levels was 75-85°C for X1, 50-70°C for X2, and 200-240 mm/s for X3, respectively. The responses studied were hardness, disintegration time, dissolution, physiochemical, and pharmacokinetic characterization. X-ray powder diffraction indicated partial or complete conversion of the crystalline drug into amorphous form in the printlets. Comparative pharmacokinetics between Viread® (generic) and printlets in rats were superimposable. Pharmacokinetic parameters showed statistically insignificant differences between the two formulations in terms of Tmax, Cmax, and AUC of (p > 0.05). Printlets were bioequivalent to Viread® as per FDA bioequivalence criteria. Thus, the SLS printing method showed the fabrication of dose-flexible printlets with quality, and in vivo performance equivalent to commercial tablets.
Keywords: Amorphous; Bioequivalence; Critical quality attributes; Pharmacokinetic; Selective laser sintering; Tenofovir disoproxil fumarate.
© 2023. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Conflict of interest statement
Figures



References
-
- UNAIDS: Global HIV and AIDS statistics–fact sheet. 2021. https://www.unaids.org/en/resources/fact-sheet. Accessed 31 May 2022.
-
- Lloyd A. HIV infection and AIDS. P N G Med J. 1996;39(3):174–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

