PERK Inhibition Suppresses Neovascularization and Protects Neurons During Ischemia-Induced Retinopathy
- PMID: 37566408
- PMCID: PMC10424802
- DOI: 10.1167/iovs.64.11.17
PERK Inhibition Suppresses Neovascularization and Protects Neurons During Ischemia-Induced Retinopathy
Abstract
Purpose: Retinal ischemia is a common cause of a variety of eye diseases, such as retinopathy of prematurity, diabetic retinopathy, and vein occlusion. Protein kinase RNA-activated-like endoplasmic reticulum (ER) kinase (PERK), one of the main ER stress sensor proteins, has been involved in many diseases. In this study, we investigated the role of PERK in ischemia-induced retinopathy using a mouse model of oxygen-induced retinopathy (OIR).
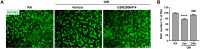
Methods: OIR was induced by subjecting neonatal pups to 70% oxygen at postnatal day 7 (P7) followed by returning to room air at P12. GSK2606414, a selective PERK inhibitor, was orally administrated to pups right after they were returned to room air once daily until 1 day before sample collection. Western blot, immunostaining, and quantitative PCR were used to assess PERK phosphorylation, retinal changes, and signaling pathways in relation to PERK inhibition.
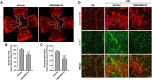
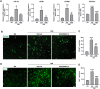
Results: PERK phosphorylation was prominently increased in OIR retinas, which was inhibited by GSK2606414. Concomitantly, PERK inhibition significantly reduced retinal neovascularization (NV) and retinal ganglion cell (RGC) loss, restored astrocyte network, and promoted revascularization. Furthermore, PERK inhibition downregulated the recruitment/proliferation of mononuclear phagocytes but did not affect OIR-upregulated canonical angiogenic pathways.
Conclusions: Our results demonstrate that PERK is involved in ischemia-induced retinopathy and its inhibition using GSK2606414 could offer an effective therapeutic intervention aimed at alleviating retinal NV while preventing neuron loss during retinal ischemia.
Conflict of interest statement
Disclosure:
Figures


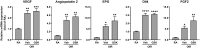
References
-
- Kermorvant-Duchemin E, Sapieha P, Sirinyan M, et al. .. Understanding ischemic retinopathies: emerging concepts from oxygen-induced retinopathy. Doc Ophthalmol. 2010; 120(1): 51–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

